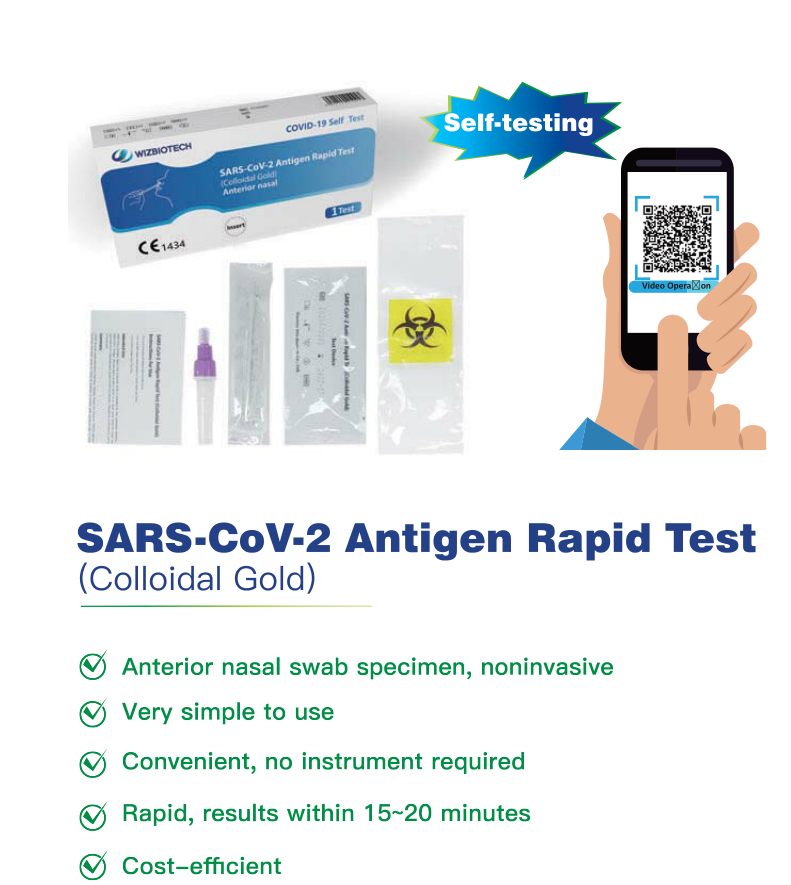
I-Mylasia igunyaze i-SARS-CoV-2 antigen yokuhlola ikhithi yokuhlola esheshayo
I-Mylasia igunyaze i-SARS-CoV-2 antigen yokuhlola ikhithi yokuhlola esheshayo
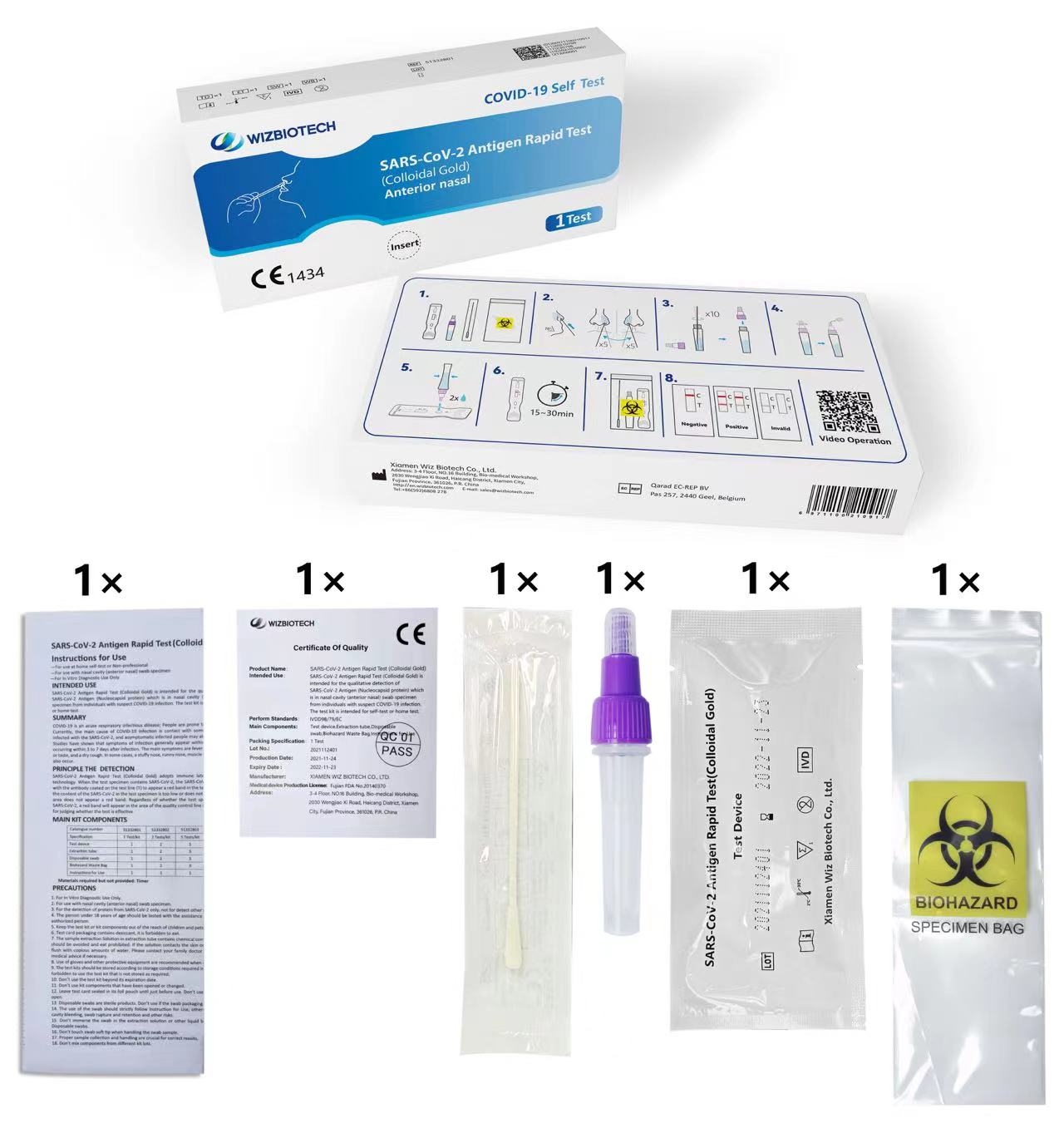
Imiyalo Yokusetshenziswa
-Izosetshenziswa ekhaya
ukuzihlola noma Ongeyena uchwepheshe
-Isetshenziswa nge-nasal cavity (anterior nasal) swab specimen
-Ngokusetshenziswa kwe-In Vitro Diagnostic Kuphela
Isitoreji
Ikhithi yokuhlola kufanele igcinwe ama-conditons angu-2°C~30°C, omile futhi angangeni elangeni (Ungawaqandisi ikhithi noma izingxenye zayo).
Isikhathi seshelufu sekhithi yizinyanga eziyi-12.
Ikhadi lokuhlola kufanele lisetshenziswe kungakapheli imizuzu engama-60 ngemuva kokuvula isikhwama se-aluminium foil.
Ukuze uthole usuku lokuphelelwa yisikhathi kwekhithi, sicela ubheke ilebula yomkhiqizo.
Ukuzwela:98.26% (95%CI 93.86%~99.79%)
Ukucaciswa:100.00% (95%CI 99.19%~100.00%)
Inani Elibikezelayo Elihle:100% (95%CI 96.79%~100.00%)
Inani Lokubikezela Okubi:99.56% (95%CI 98.43%~99.95%)
Isivumelwano Sephesenti Sisonke:99.65% (95%CI 98.74~99.96%)
I-SARS-CoV-2 Antigen Rapid Test ihloselwe ukutholwa kwekhwalithi ye-SARS-CoV-2 Antigen ku-oropharyngea swab kanye ne-nasopharyngeal swab specimens ku-Vitro.