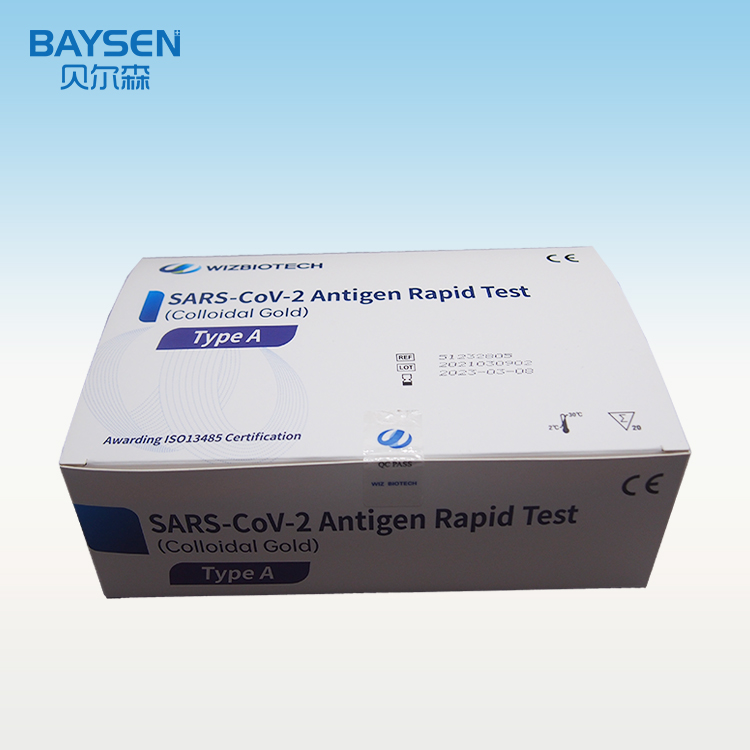
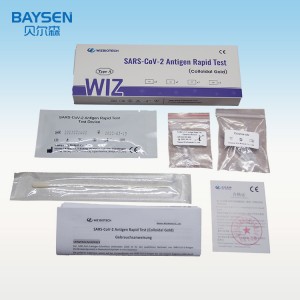
Ukuhlolwa kokusetshenziswa kwasekhaya kwe-COVID-19 nasal antigen
I-SARS-CoV-2 Antigen Rapid Test (Colloidal Gold) ihloselwe ukutholwa kwekhwalithi ye-SARS-CoV-2 Antigen(Nucleocapsid protein) ezibonelweni ze-nasal swab in vitro. Imiphumela emihle ibonisa ukuba khona kwe-SARS-CoV-2 antigen. Kufanele kuphinde kuxilongwe ngokuhlanganisa umlando wesiguli kanye nolunye ulwazi lokuxilonga[1]. Imiphumela emihle ayibandakanyi ukutheleleka ngebhaktheriya noma okunye ukutheleleka ngegciwane. Amagciwane atholakele awawona ngempela imbangela eyinhloko yezimpawu zesifo. Imiphumela engemihle ayibandakanyi ukutheleleka kwe-SARS-CoV-2, futhi akufanele kube ukuphela kwesisekelo sezinqumo zokwelashwa noma zokuphatha isiguli (okuhlanganisa nezinqumo zokulawula ukutheleleka). Naka umlando wokuxhumana wesiguli wakamuva, umlando wezokwelapha kanye nezimpawu nezimpawu ezifanayo ze-COVID-19, uma kunesidingo, kuyatuswa ukuthi uqinisekise lawa masampula ngokuhlolwa kwe-PCR ukuze uphathe isiguli. Okwabasebenzi baseselabhorethri abathole isiqondiso noma ukuqeqeshwa futhi abanolwazi lochwepheshe lokuxilongwa kwe-in vitro, nakubasebenzi abafanelekile abaye bathola ukulawula ukutheleleka noma ukuqeqeshwa kobuhlengikazi[2].