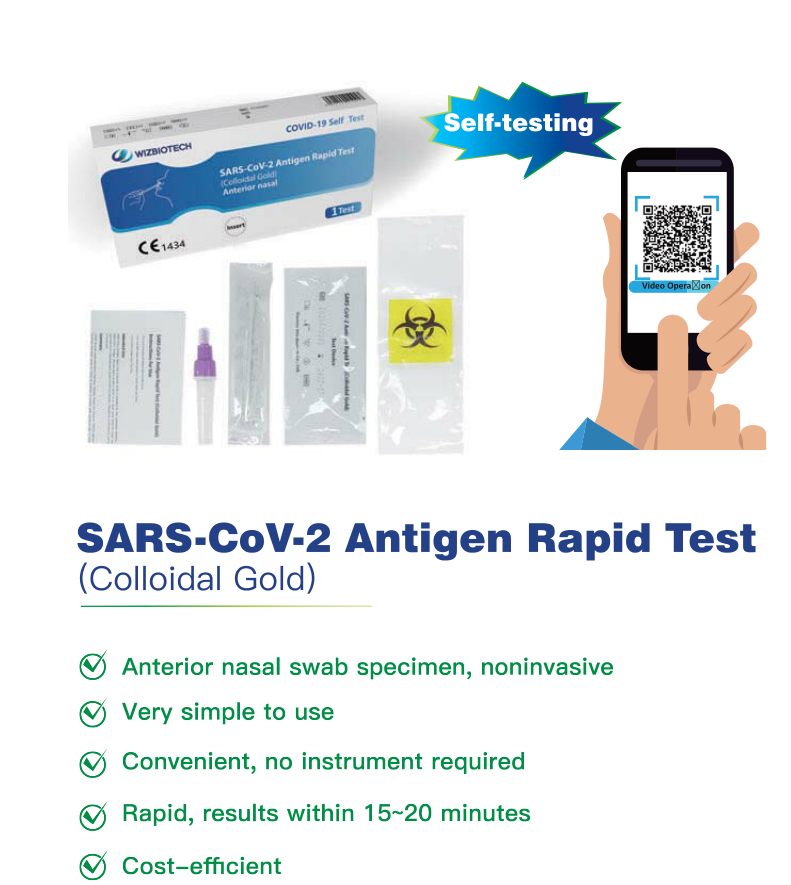
I-Mylasia ivume i-SARS-CoV-2 i-antigen yokuzivavanya ngokukhawuleza kwikhithi yokuzivavanya
I-Mylasia ivume i-SARS-CoV-2 i-antigen yokuzivavanya ngokukhawuleza kwikhithi yokuzivavanya
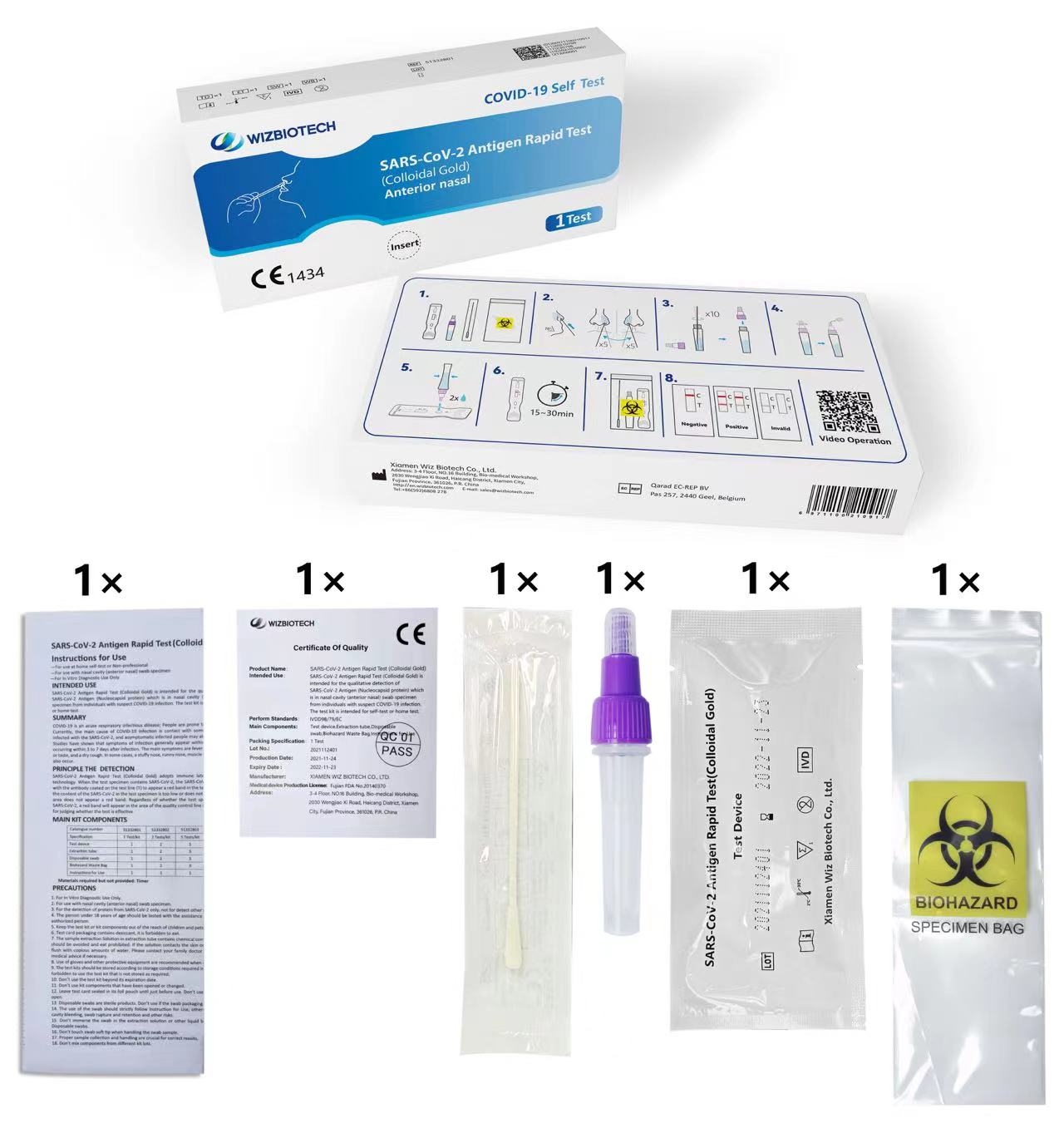
Imiyalelo yokusetyenziswa
—Izakusetyenziswa ekhaya
ukuzivavanya okanye Non-professional
-Ukusetyenziswa kumngxuma weempumlo (umphambili wempumlo) umfuziselo weswab
—Kukusetyenziswa kwe-In Vitro Diagnostic Kuphela
Ugcino
Ikhithi yovavanyo kufuneka igcinwe kwi-conditons ye-2 ° C ~ 30 ° C, yomile kwaye ngaphandle kwelanga elithe ngqo (Musa ukuyikhenkceza ikiti okanye izinto zayo).
Ubomi beshelufu bekiti ziinyanga ezili-12.
Ikhadi lovavanyo kufuneka lisetyenziswe kwimizuzu engama-60 emva kokuvula isikhwama se-aluminium foil.
Ngomhla wokuphelelwa kwekhithi, nceda ubhekisele kwileyibhile yemveliso.
Uvakalelo: 98.26% (95% CI 93.86% ~ 99.79%)
Ucalulo:100.00% (95%CI 99.19%~100.00%)
Ixabiso Eliqikelelwayo Eliqinisekileyo: 100% (95% CI 96.79% ~ 100.00%)
Ixabiso lokuQikelela okuNegativity: 99.56% (95% CI 98.43% ~ 99.95%)
IsiVumelwano seepesenti xa sisonke: 99.65% (95% CI 98.74~99.96%)
I-SARS-CoV-2 Antigen Rapid Test yenzelwe ukuchongwa okusemgangathweni kwe-SARS-CoV-2 Antigen kwi-oropharyngea swab kunye ne-nasopharyngeal swab specimens kwi-Vitro.