Wholesale Enterovirus 71 - Diagnostic Kit (Colloidal Gold) for Dengue NS1 Antigen – Baysen
Wholesale Enterovirus 71 - Diagnostic Kit (Colloidal Gold) for Dengue NS1 Antigen – Baysen Detail:
Diagnostic Kit (Colloidal Gold) for Dengue NS1 Antigen
For in vitro diagnostic use only
Please read this package insert carefully prior to use and strictly follow the instructions. Reliability of assay results cannot be guaranteed if there are any deviations from the instructions in this package insert.
INTENDED USE
Diagnostic Kit(Colloidal Gold)for Dengue NS1 Antigen is a colloidal gold immunochromatographic assay for the qualitative detection of Dengue NS1 Antigen in human whole blood, serum or plasma, which is used for clinical diagnosis of dengue infection.This test is a screening reagent. All positive sample must be confirmed by other methodologies. This test is intended for healthcare professional use only.
PACKAGE SIZE
Twenty-five serving.
SUMMARY
Dengue Fever (Dengue Fever) is caused by Dengue virus infection. Dengue Fever is a group B vector virus and the yellow virus family flavivirus. Dengue virus antibody tests are often used enzyme-linked Immunoassay, Indirect Immunofluorescent Assay, Immunochromato – graphic Test. In recent years, with the development of the immune colloidal gold chromatography technology, using colloidal gold immune chromatography to detect serum (plasma) dengue virus antibody has become a reality. This method is characterized by simple operation, quick detection speed and saving manpower and material resources. It makes up for the inconveniences of ELISA and chemiluminescence method.
ASSAY PROCEDURE
1.Take out the test card from the foil bag, put it on the level table and mark it.
2. Add 120μl(about 3 drop) serum or plasma sample or 35ul(about 1 drop) whole blood sample to sample well of the card with provided dispette, then add 2 drop sample diluents; start timing;
3. The result should be read within 10-20minutes, and it is invalid after 20 minutes.
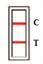
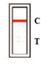
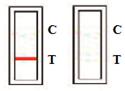
TEST RESULTS AND INTERPRETATION[2]
Product detail pictures:

Related Product Guide:
Cardiac Biomarkers Market To Reach $13.3 Billion By 2024: Grand View Research, Inc. | Psa Test Cost
Rs 60 test detects early-stage prostate cancer | Calprotectin Elisa Kit
abide by the contract", conforms to the market requirement, joins in the market competition by its high quality as well as provides more comprehensive and excellent service for clients to let them become big winner. The pursue of the company, is the clients' satisfaction for Wholesale Enterovirus 71 - Diagnostic Kit (Colloidal Gold) for Dengue NS1 Antigen – Baysen , The product will supply to all over the world, such as: Slovakia, Detroit, Amman, Our products have mainly exported to south-east Asia Euro-America, and sales to all of our country. And depending on excellent quality, reasonable price, best service, we've got got good feedback from customers overseas. You are welcomed to join us for more possibilities and benefits. We welcome customers, business associations and friends from all parts of the world to contact us and seek cooperation for mutual benefits.
The sales manager is very patient, we communicated about three days before we decided to cooperate, finally, we are very satisfied with this cooperation!