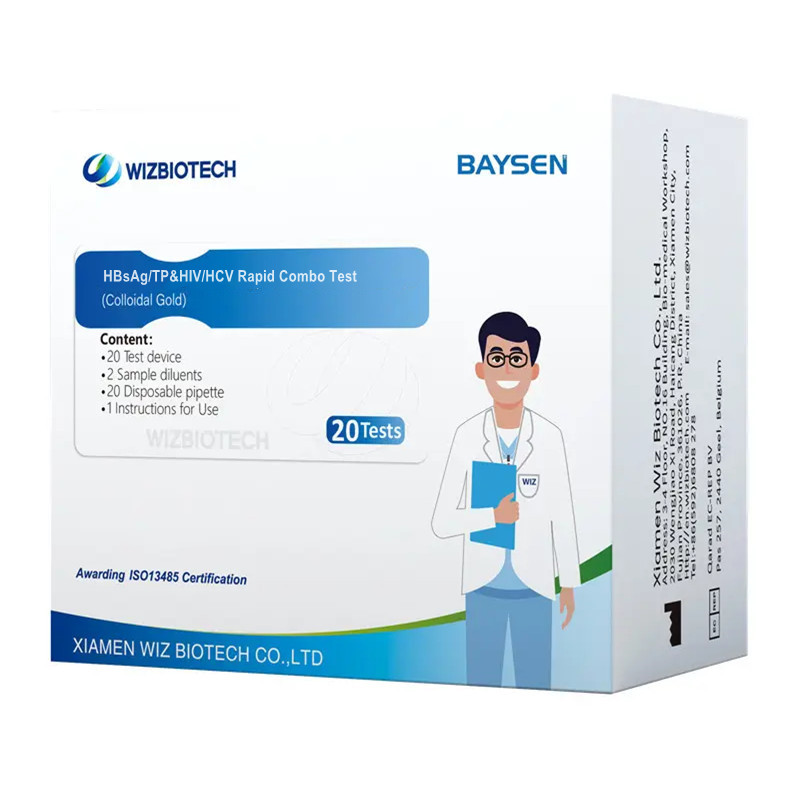
Inotapukira HIV HCV HBSAG UYE Syphilish Rapid Combo Test
MADZIDZO EKUGADZIRA
| Model Number | HBsAg/TP&HIV/HCV | Packing | 20 Miedzo / kit, 30kits / CTN |
| Zita | HBsAg/TP&HIV/HCV Rapid Combo Test | Instrument classification | Kirasi III |
| Features | High senitivity, Easy operation | Chitupa | CE/ ISO13485 |
| Kururama | > 97% | Sherufu hupenyu | Makore maviri |
| Methodology | Colloidal Gold | OEM / ODM sevhisi | Avaliable |
Superiority
Nguva yekuedza: 15-20mins
Kuchengetedza:2-30℃/36-86℉
Nzira: Colloidal Gold
Feature:
• High sensitive
• mhedzisiro kuverenga mumaminitsi 15-20
• Easy operation
• Kururama kwepamusoro
ZVIRI KUSHANDISA
Iyi kit inokodzera in vitro qualitative determination of hepatitis B virus, syphilis spirochete, human immunodeficiency virus, uye hepatitis C muserum yevanhu/plas-.ma/samples eropa rekuwedzera kuongororwa kwehutachiona hwehepatitis B, syphilis spirochete, human immunodeficiency virus, uye hutachiona hwehepatitis C. Zvigumisiro zvakawanikwa zvinofanirakuongororwa pamwe chete nemamwe mashoko ekiriniki. Inoitirwa kushandiswa nenyanzvi dzekurapa chete.
Test maitiro
| 1 | Verenga rairo yekushandiswa uye mukunyatso kuenderana nekuraira kwekushandiswa kunodiwa kudzivirira kukanganisa kurongeka kwemhedzisiro yebvunzo. |
| 2 | Pamberi pekuyedzwa, kiti uye sampu inotorwa kubva munzvimbo yekuchengetera uye yakaenzana kusvika kune tembiricha yekamuri womaka. |
| 3 | Kubvarura kurongedzerwa kwealuminium foil pouch, buritsa mudziyo wekuyedza womaka, wobva waiisa yakachinjika patafura yekuyedza. |
| 4 | Aspirate serum / plasma samples ine donhwe rinoraswa uye wedzera madonhwe maviri mune imwe neimwe yematsime s1 uye s2; wedzera madonhwe matatu mune rimwe nerimwe rematsime s1 uye s2 eropa rese resampuli usati wawedzera madonhwe 1 ~ 2 emushonga wekugezesa kune rimwe nerimwe rematsime s1 uye s2 uye Nguva inotanga. |
| 5 | Mhinduro dzebvunzo dzinofanirwa kududzirwa mukati me15 ~ 20 maminetsi, kana anopfuura maminetsi makumi maviri akaturikirwa mhinduro isiri iyo. |
| 6 | Kupirikira kunoonekwa kunogona kushandiswa mukududzira kwemhedzisiro. |
Ongorora: imwe neimwe sampu ichaputirwa nepipette yakachena inoraswa kudzivirira kusvibiswa kwemuchinjiko.
KLINICAL PERFORMANCE
| WIZ Mibairo yeHBsag
| Muedzo weReference reagent | Yakanaka masangana mwero: 99.06% (95% CI 96.64% ~ 99.74%) Negative coincidence rate: 98.69% (95% CI96.68% ~ 99.49%) Yese masangana mwero:98.84% (95%CI97.50%~99.47% | ||
| Positive | Negative | Total | ||
| Positve | 211 | 4 | 215 | |
| Negative | 2 | 301 | 303 | |
| Total | 213 | 305 | 518 | |
| WIZ Mibairo yeTP
| Muedzo weReference reagent | Yakanaka masangana mwero: 96.18% (95% CI 91.38% ~ 98.36%) Negative coincidence rate: 97.67% (95% CI95.64% ~ 98.77%) Yese masangana mwero: 97.30% (95% CI95.51% ~ 98.38%) | ||
| Positive | Negative | Total | ||
| Positve | 126 | 9 | 135 | |
| Negative | 5 | 378 | 383 | |
| Total | 131 | 387 | 518 | |
| WIZ Mibairo yeHCV
| Muedzo weReference reagent | Yakanaka masangana mwero: 93.44% (95% CI 84.32%~97.42%) Negative coincidence rate: 99.56% (95% CI98.42% ~ 99.88%) Yese masangana mwero:98.84% (95%CI97.50%~99.47%) | ||
| Positive | Negative | Total | ||
| Positve | 57 | 2 | 59 | |
| Negative | 4 | 455 | 459 | |
| Total | 61 | 457 | 518 | |
| WIZ Mibairo yeHIV
| Muedzo weReference reagent | Yakanaka masangana mwero: 96.81% (95%CI 91.03%~98.91%) Negative coincidence rate: 99.76% (95% CI98.68% ~ 99.96%) Yese masangana mwero: 99.23% (95%CI98.03%~99.70%) | ||
| Positive | Negative | Total | ||
| Positve | 91 | 1 | 92 | |
| Negative | 3 | 423 | 446 | |
| Total | 94 | 424 | 518 | |