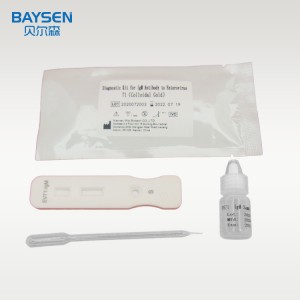
IgM antibody Enterovirus 71 EV71 yekukurumidza test kit EV 71 antibody
Products Parameters


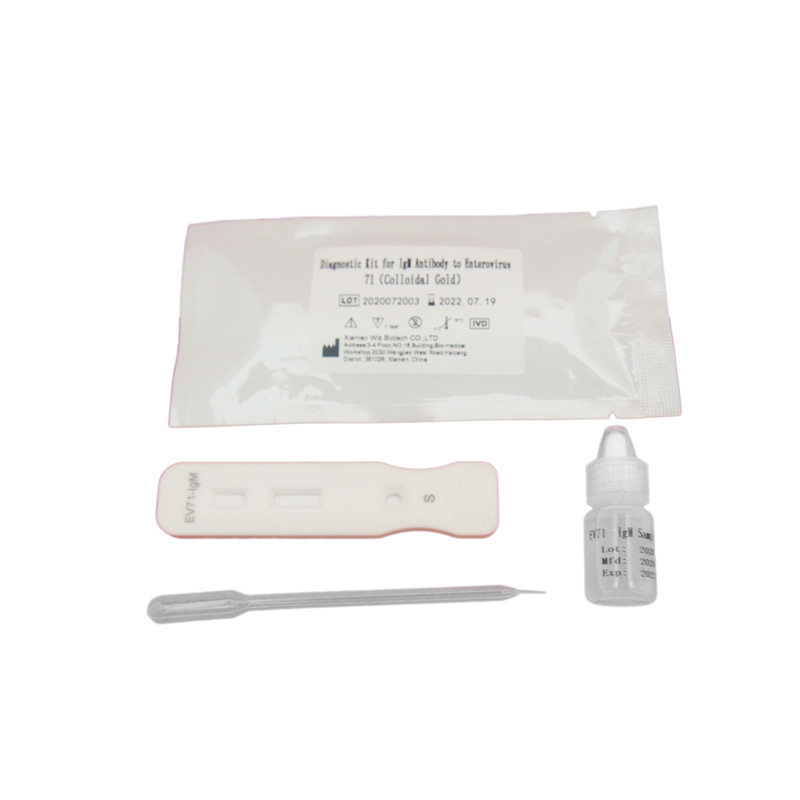
ZVINHU UYE MAITIRO YEFOB TEST
PRINCIPLE
Iyo membrane yechishandiso chekuyedza yakavharwa ne anti EV71 antibody padunhu rebvunzo uye mbudzi anti tsuro IgG antibody panzvimbo yekutonga. Lable pad yakaputirwa nefluorescence yakanyorwa anti EV71 antibody uye tsuro IgG pamberi. Paunenge uchiyedza sampuli yakanaka, iyo EV71 antigen mumuenzaniso inosanganisa nefluorescence yakanyorwa anti EV71 antibody, uye gadzira immune musanganiswa. Pasi pechiito chechromatography, kuyerera kwakaoma kwakananga kune bepa rinonyudza, apo yakaoma yakapfuura dunhu rekuyedza, yakasanganiswa neAnti EV71 yekuputira antibody, inoumba nyowani yakaoma.
Kana zvisina kunaka, sampuli haina enterovirus 71 IgM antibody, kuitira kuti immune yakaoma irege kuumbwa. Pachave pasina mutsara mutsvuku munzvimbo yekuona (T). Hazvina mhosva kana Enterovirus 71 IgM antibody iripo mumuenzaniso kana kuti kwete, iyo yakasara colloidal yegoridhe-yakanyorwa mbeva inorwisa munhu IgM monoclonal antibody uye mbudzi anti-mbeva IgG antibody yakavharwa munzvimbo yekudzora mhando (C) inosunga. Ipapo iyo agglutinates inovandudza ruvara munzvimbo yekudzora yemhando, uye mutsara mutsvuku uchaonekwa mu (C). Mutsara mutsvuku ndiwo chiyero chinoonekwa munharaunda yekudzora yemhando (C) yekutonga kana paine masampuli akakwana uye kana iyo chromatography process yakajairika. Inoshandiswawo seyemukati yekudzora chiyero che reagents.
Test Procedure:
1.Mienzaniso yakaedzwa inogona kuva ropa rose, kusanganisira ropa re venous kana Peripheral blood. Ropa rose harigone kuchengetwa mushure mekuunganidzwa. Ndinofanira kushandiswa nokukurumidza mushure mekuunganidza.
2.Serum samples inounganidzwa septically maererano nemaitiro akajairika. Kupisa-inactivated serum haigone kushandisa. Hazvikurudzirwi kushandisa lipemic, turbid kana serum yakasvibiswa. Particulate nyaya mu serum. Uye kunaya kuchakanganisa mhinduro dzebvunzo, masampuli akadai anofanirwa kuiswa centrifuged kana kusefa asati ashandiswa.
3.Mienzaniso yakaedzwa inogona kuva heparin, Sodium citrate kana EDTA anticoagulant plasma.
4.According to standard techniques unganidza sampuli. Serum kana plasma sampuli inogona kuchengetwa mufiriji pa 2-8 ℃ kwemazuva matatu uye cryopreservation pazasi -15 ° C kwemwedzi mitatu.
5.Yese sampuli inodzivirira kutonhora-kunyungudika kutenderera.

Nezvedu

Xiamen Baysen Medical Tech yakaganhurirwa ibhizinesi repamusoro rebiological iro rinozvipira kufaira rekukurumidza diagnostic reagent uye rinobatanidza kutsvagisa nekusimudzira, kugadzira uye kutengesa mune yakazara. Kune akawanda epamberi ekutsvagisa tsvimbo uye maneja ekutengesa mukambani, vese vane ruzivo rwakapfuma rwekushanda muchina uye kune dzimwe nyika biopharmaceutical bhizinesi.
Chitupa kuratidza