8 Makore Anotengesera China Yakarurama HCV Yekukurumidza Diagnostic Test Rapid Test Kits muPathological Analysis Equipments
Isu tinonakidzwa nechimiro chakanyanya kunaka pakati petarisiro yedu yezvigadzirwa zvedu zvemhando yepamusoro, mutengo wemakwikwi uye sevhisi yakakodzera ye8 Makore Exporter China Yakarurama.HCV Rapid Diagnostic TestRapid Test Kits muPathological Analysis Equipments, Takazvishingisa pachedu kuti kuchaonekwa sekuuya kwakavimbisa uye tinovimba tinogona kuve nekushandira pamwe kwenguva refu netarisiro kubva kunharaunda yese.
Isu tinonakidzwa nechimiro chakanyanya kunaka pakati petarisiro yedu yezvigadzirwa zvedu zvemhando yepamusoro, mutengo wemakwikwi uye sevhisi yakakodzeraChina Rapid Test, HCV Rapid Diagnostic Test, Zvinhu zvedu zvakakurumbira mushoko, seSouth America, Africa, Asia uye zvichingodaro. Makambani "kugadzira zvigadzirwa zvekutanga-kirasi" sechinangwa, uye kuyedza kupa vatengi mhinduro dzemhando yepamusoro, kupa emhando yepamusoro mushure mekutengesa sevhisi uye tsigiro yehunyanzvi, uye kubatsirana kwevatengi, kugadzira basa riri nani uye ramangwana!
Yekushandisa in vitro diagnostic chete
Ndokumbira uverenge iyi pasuru isa nekungwarira usati washandisa uye kunyatsotevera mirairo. Kuvimbika kwemhedzisiro yeassay hakugone kuvimbiswa kana paine chero kutsauka kubva kune mirairo mune ino pasuru yekuisa.
ZVIRI KUSHANDISA
Diagnostic Kit yeHepatitis C Virus Antibody (Fluorescence Immunochromatographic Assay) is a fluorescence immunochromatographic assay yehuwandu hwekuonekwa kweHCV antibody muserum yemunhu kana plasma, iyo yakakosha kuongororwa kukosha kwechirwere chehepatitis C. Yese sampuli yakanaka inofanira kusimbiswa neimwe nzira. Muedzo uyu wakaitirwa kushandiswa nenyanzvi dzezvehutano chete
1.Kuisa parutivi zvose reagents uye samples kune tembiricha yekamuri.
2.Vhura iyo Portable Immune Analyzer (WIZ-A101), isa iyo password password login maererano nenzira yekushanda yechiridzwa, uye pinda iyo yekuona interface.
3.Scan dentification code kuti usimbise chinhu chekuedza.
4.Bvisa kadhi rekuedza kubva mubhegi refoil.
5.Isai kadhi rekuedza mukadhi yekadhi, tarisai QR code, uye sarudza chinhu chekuedza.
6.Wedzera 20μL serum kana plasma sampuli kuti sampuli diluent, uye sanganisa zvakanaka.
7.Wedzera 80μL sampuli mhinduro kuti uenzanise zvakanaka wekadhi.
8.Click "standard test" bhatani, mushure memaminitsi e15, chiridzwa chichangoona kadhi rekuedza, rinogona kuverenga migumisiro kubva pachiratidziro chechiratidzo chechiridzwa, uye nyora / kudhinda mhinduro dzekuedza.
9.Rejera kune rairo yePortable Immune Analyzer(WIZ-A101).
SUMMARY
Hepatitis C virus (HCV) ihamvuropu, single stranded positive sense RNA (9.5 kb) utachiona hwemhuri yeFlaviviridae. Matanhatu makuru genotypes uye akatevedzana e subtypes eHCV akaonekwa. Yakaparadzaniswa muna 1989, HCV zvino yava kuzivikanwa sechisakiso chikuru chokuisira ropa chine chokuita nenon-A, non-B hepatitis. Chirwere ichi chinoratidzwa neacute uye chisingaperi chimiro. Vanopfuura 50% vevanhu vane hutachiwana vanokura zvakanyanya, zvinotyisidzira upenyu hepatitis ine chiropa cirrhosis uye hepatocellular carcinomas. Kubva pakatanga kushandiswa muna 1990 kuongorora ropa kunorwisa HCV, kuwanda kwechirwere ichi kune vanowedzerwa ropa kwakaderedzwa zvikuru. Zvidzidzo zvekiriniki zvinoratidza kuti huwandu hwakakosha hwevanhu vane HCV vanogadzira masoja ekudzivirira chirwere kuNS5 isiri-yakarongeka protein yehutachiona. Kune izvi, bvunzo dzinosanganisira maantigen kubva kuNS5 nharaunda yehutachiona genome kuwedzera kune NS3 (c200), NS4 (c200) uye iyo Core (c22).
ZVINOTAURWA ZVINOITA
Iyo membrane yechishandiso chekuyedza yakavharwa neHCV antigen padunhu rekuyedza uye mbudzi anti tsuro IgG antibody padunhu rekutonga. Lable pad yakaputirwa nefluorescence yakanyorwa HCV antigen uye tsuro IgG pamberi. Paunenge uchiyedza sampuli yakanaka, iyo HCV antibody mumuenzaniso inosanganisa nefluorescence yakanyorwa HCV antigen, uye gadzira musanganiswa wekudzivirira muviri. Pasi pechiito che immunochromatography, iyo yakaoma kuyerera munzira yeanononzva bepa, apo yakaoma yakapfuura dunhu rekuyedzwa, yakasanganiswa neHCV antigen coating antigen, inoumba nyowani complex.HCV antibody level inoenderana neiyo fluorescence chiratidzo, uye kuwanda kweHCV antibody musampuli inogona kuwonekwa ne fluorescence immunoassay assay.
REAGENTS NEZVINHU ZVINOPIWA
25T package zvikamu:
.Kuedza kadhi rimwe nerimwe foil rakaiswa muhomwe ine desiccant
.Sample diluents
.Package isa
ZVINHU ZVINODIWA ASI ZVISINA KUPIWA
Sample yekuunganidza mudziyo, timer
SAMPLE KUCHENGA UYE KUCHENGA
1.Mienzaniso yakaedzwa inogona kuva serum, heparin anticoagulant plasma kana EDTA anticoagulant plasma.
2.According to standard techniques unganidza sampuli. Serum kana plasma sampuli inogona kuchengetwa firiji pa 2-8 ℃ kwemazuva 7 uye cryopreservation pazasi -15 ° C kwemwedzi mitanhatu.
3.Yese sampuli inodzivirira kutonhora-kunyungudika kutenderera.
ASAY PROCEDURE
Ndokumbira uverenge bhuku rekushandisa chekushandisa uye pasuru isa usati waedza.
.Ichi mhedzisiro yebvunzo ndeyekuongororwa kwekiriniki chete, haifanire kushanda seyo chete hwaro hwekuongororwa kwekiriniki uye kurapwa, varwere vekiriniki manejimendi inofanirwa kutariswa yakazara yakasanganiswa nezviratidzo zvayo, nhoroondo yezvekurapa, mamwe ongororo yerabhoritari, mhinduro yekurapa, epidemiology uye mamwe mashoko.
.Iri reagent rinoshandiswa chete serum uye plasma tests. Iyo inogona kusawana mhedzisiro chaiyo kana ichishandiswa kune mamwe masampuli senge mate uye weti uye nezvimwe.
PERFORMANCE CHARACTERISTICS
| Linearity | 0.005-5 | hama kutsauka: -15% kusvika +15%. |
| Linear coefficient yekubatanidza:(r)≥0.9900 | ||
| Kururama | Chiyero chekudzorera chichave mukati me85% - 115%. | |
| Kudzokorora | CV≤15% | |
REFERENCES
1.Post transfusion hepatitis. Mu: Moore SB, ed. Transfusion-Transmitted Viral Diseases. Alington, VA. Am. Assoc. Mabhangi Eropa, mapeji 53-38.
2.Hansen JH, et al.HAMA Kupindira neMurine Monoclonal Antibody-Based Immunoassays[J].J yeClin Immunoassay,1993,16:294-299.
3.Levinson SS.Mamiriro eHeterophilic Antibodies uye Basa muImmunoassay Interference[J].J yeClin Immunoassay,1992,15:108-114.
4.Alter HJ., Purcell RH, Holland PV, et al. (1978) Transmissible mumiririri mune isiri-A, isiri-B hepatitis. Lancet I: 459-463.
5.Choo QL,Weiner AJ, Overby LR, Kuo G, Houghton M. (1990) Hepatitis C Virus: iyo huru inokonzeresa yehutachiwana isiri A, isiri B hepatitis. Br Med Bull 46: 423-441.
6.Engvall E, Perlmann P. (1971) Enzyme yakabatanidzwa immunosorbent assay (ELISA): qualitative assay yeIgG. Immunochemistry 8:871-874.
ZVINOTARISIRWA ZVINOKOSHA
HCV-Ab <0.02
Zvinokurudzirwa kuti murabhoritari yega yega imise zera rayo rakajairwa rinomiririra huwandu hwevarwere vayo.
TEST RESULTS UYE DUDZIRIRO
- Idzi data riri pamusoro ndiro mhedzisiro yeHCV-Ab reagent bvunzo, uye zvinokurudzirwa kuti murabhoritari yega yega inofanirwa kumisa huwandu hweHCV-Ab yekuona kukosha kwakakodzera huwandu hwevanhu mudunhu rino. Mibairo iri pamusoro ndeyekutarisa chete.
- Migumisiro yeiyi nzira inongoshanda kune mitsara yereferensi yakagadzwa nenzira iyi, uye hapana kuenzaniswa kwakananga nedzimwe nzira.
- Zvimwe zvinhu zvinogona kukonzera kukanganisa mumhedzisiro yekuona, kusanganisira zvikonzero zvehunyanzvi, zvikanganiso zvekushanda uye zvimwe zvinhu zvemuenzaniso.
KUCHENGA UYE KUTSIGA
- Iyo kit ndeye 18 mwedzi pasherufu-hupenyu kubva pazuva rekugadzirwa. Chengetedza makiti asina kushandiswa pa2-30°C. MUSAIZE. Usashandise kupfuura zuva rekupera.
- Usavhure homwe yakavharwa kusvika wagadzirira kuita bvunzo, uye bvunzo-yekushandisa imwe chete inokurudzirwa kuti ishandiswe pasi penzvimbo inodiwa (tembiricha 2-35 ℃, humidity 40-90%) mukati me60 mins nekukurumidza sezvinobvira.
- Sample diluent inoshandiswa pakarepo mushure mekuvhurwa.
YAMBIRO UYE CHENJERERO
.Ikiti inofanira kuvharwa uye kuchengetedzwa kubva pakunyorova.
.Yese mienzaniso yakanaka ichasimbiswa nedzimwe nzira.
.Mienzaniso yese inofanira kubatwa seinogona kusvibisa.
.USASHANDISE expired reagent.
.USACHINANA mareagents pakati pemakiti ane akasiyana lot Nha.
.USASHANDISE zvakare makadhi ebvunzo uye chero zvinhu zvinoraswa.
.Misoperation, yakawandisa kana diki sampuli inogona kukonzeresa kukanganisa.
LIMITATION
.Sezvinoita chero bvunzo inoshandisa masoja ekudzivirira mbeva, mukana uripo wekukanganiswa nemasoja ekudzivirira mbeva (HAMA) mumuenzaniso. Mienzaniso kubva kuvarwere vakagamuchira gadziriro dzemonoclonal antibodies yekuongorora kana kurapwa inogona kunge iine HAMA. Mienzaniso yakadaro inogona kukonzera nhema dzakanaka kana nhema dzisina kunaka.
Kiyi yezviratidzo zvakashandiswa:
 | In Vitro Diagnostic Medical Device |
 | Mugadziri |
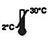 | Chengetedza pa2-30 ℃ |
 | Zuva rekupera |
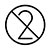 | Usashandisezve |
 | KUCHENJERA |
 | Bvunza Mirayiridzo Yekushandisa |