Renewable Design for CE approved monkeypox test - Diagnostic Kit for Cardiac Troponin I Myoglobin and Isoenzyme MB of Creatine Kinase – Baysen
Renewable Design for CE approved monkeypox test - Diagnostic Kit for Cardiac Troponin I Myoglobin and Isoenzyme MB of Creatine Kinase – Baysen Detail:
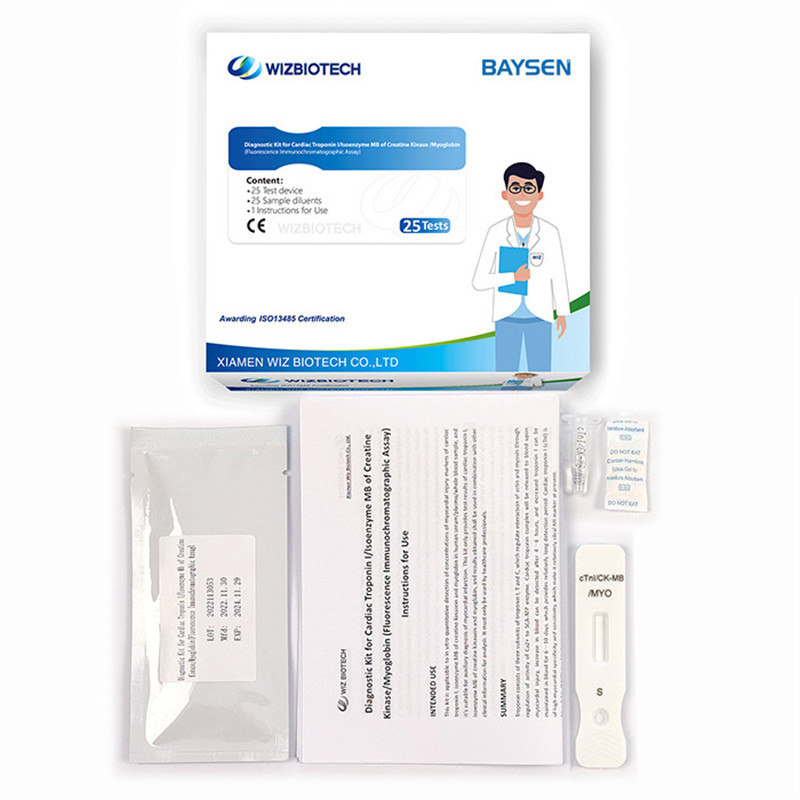
Diagnostic Kit for Cardiac Troponin I ∕Isoenzyme MB of Creatine Kinase ∕Myoglobin
Methodology:Fluorescence Immunochromatographic Assay
Production information
| Model Number | cTnI/CK-MB/MYO | Packing | 25 Tests/ kit, 30kits/CTN |
| Name | Diagnostic Kit for Cardiac Troponin I ∕Isoenzyme MB of Creatine Kinase ∕Myoglobin | Instrument classification | Class II |
| Features | High sensitivity, Easy opeation | Certificate | CE/ ISO13485 |
| Accuracy | > 99% | Shelf life | Two Years |
| Methodology | Fluorescence Immunochromatographic Assay | OEM/ODM service | Avaliable |
INTENDED USE
This kit is applicable to in vitro quantitative detection of concentrations of myocardial injury markers of cardiac
troponin I, isoenzyme MB of creatine kinasein and myoglobin in human serum/plasma/whole blood sample, and
it’s suitable for auxiliary diagnosis of myocardial infarction. This kit only provides test results of cardiac troponin I,
isoenzyme MB of creatine kinasein and myoglobin, and results obtained shall be used in combination with other
clinical information for analysis. It must only be used by healthcare professionals.
Test procedure
| 1 | Before using the reagent,read the package insert carefully and familiarize yourself with the operating procedures. |
| 2 | Select standard test mode of WIZ-A101 portable immune analyzer |
| 3 | Open the aluminum foil bag package of reagent and take out the test device. |
| 4 | Horizontally insert the test device into the slot of immune analyzer. |
| 5 | On home page of operation interface of immune analyzer, click “Standard” to enter test interface. |
| 6 | Click “QC Scan” to scan the QR code on inner side of the kit; input kit related parameters into instrument and select sample type. Note: Each batch number of the kit shall be scanned for one time. If the batch number has been scanned, then skip this step. |
| 7 | Check the consistency of “Product Name”, “Batch Number” etc. on test interface with information on the kit label. |
| 8 | Take out sample diluent upon consistent information, add 80μL serum/plasma/whole blood sample, and thoroughly mix them; |
| 9 | Add 80µL aforesaid thoroughly mixed solution into well of test device; |
| 10 | After complete sample addition, click “Timing” and remaining test time will be automatically displayed on the interface. |
| 11 | Immune analyzer will automatically complete test and analysis when test time is reached. |
| 12 | After test by immune analyzer is completed, test result will be displayed on test interface or can be viewedthrough “History” on home page of operation interface. |
Note: each sample shall be pipetted by clean disposable pipette to avoid cross contamination.
Product detail pictures:


Related Product Guide:
New Study Shows MDxHealth’s SelectMDx Test is Cost-Effective for Biopsy Selection Brussels Stock Exchange:MDXH | Psa Test Cost
Cardiac Biomarkers Market To Reach $13.3 Billion By 2024: Grand View Research, Inc. | Cpn-Igm
We pursue the management tenet of "Quality is remarkable, Company is supreme, Name is first", and will sincerely create and share success with all clientele for Renewable Design for CE approved monkeypox test - Diagnostic Kit for Cardiac Troponin I Myoglobin and Isoenzyme MB of Creatine Kinase – Baysen , The product will supply to all over the world, such as: Surabaya, United States, Japan, Based on our automatic production line, steady material purchase channel and quick subcontract systems have been built in mainland China to meet customer's wider and higher requirement in recent years. We are looking forward to cooperating with more clients worldwide for common development and mutual benefit!Your trust and approval are the best reward for our efforts. Keeping honest, innovative and efficient, we sincerely expect that we can be business partners to create our brilliant future!
The company can think what our think, the urgency of urgency to act in the interests of our position, can be said this is a responsible company, we had a happy cooperation!