Quality Inspection for One Step Medical Diagnostic Rapid Fob Tumor Marker Test - Luteinizing Hormone LH Ovulation Rapid Test Kit women pregnancy detection – Baysen
Quality Inspection for One Step Medical Diagnostic Rapid Fob Tumor Marker Test - Luteinizing Hormone LH Ovulation Rapid Test Kit women pregnancy detection – Baysen Detail:
INTENDED USE
Diagnostic Kit for Luteinizing Hormone (fluorescence immunochromatographic assay) is a fluorescence immunochromatographic assay for the quantitative detection of Luteinizing Hormone (LH) in human serum or plasma, which is mainly used in the evaluation of pituitary endocrine function.All positive sample must be confirmed by other methodologies. This test is intended for healthcare professional use only.
SUMMARY
Product detail pictures:


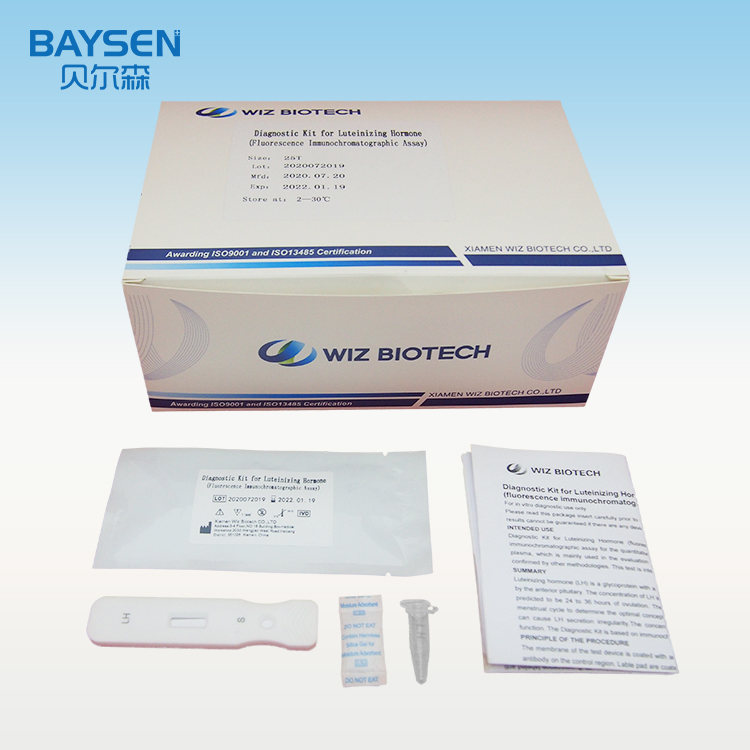
Related Product Guide:
It’s time for a new Europe-wide strategy on prostate cancer | Diagnostic Kit For Isoenzyme Mb Of C Reatine Kinase
Bolton will make money from player sales available to Phil Parkinson | P24 Test Strips
It is a great way to further improve our products and repair. Our mission is always to create innovative products to prospects with a superior expertise for Quality Inspection for One Step Medical Diagnostic Rapid Fob Tumor Marker Test - Luteinizing Hormone LH Ovulation Rapid Test Kit women pregnancy detection – Baysen , The product will supply to all over the world, such as: Detroit, United Arab emirates, Manchester, Customer's satisfaction is always our quest, creating value for customers is always our duty, a long term mutual-beneficial business relationship is what we are doing for. We are an absolutely reliable partner for you in China. Of course, other services, like consulting, can be offered too.
This company can be well to meet our needs on product quantity and delivery time, so we always choose them when we have procurement requirements.












