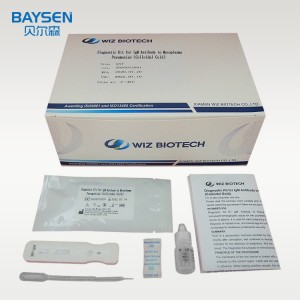
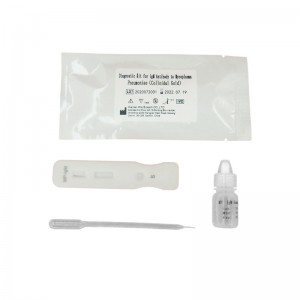
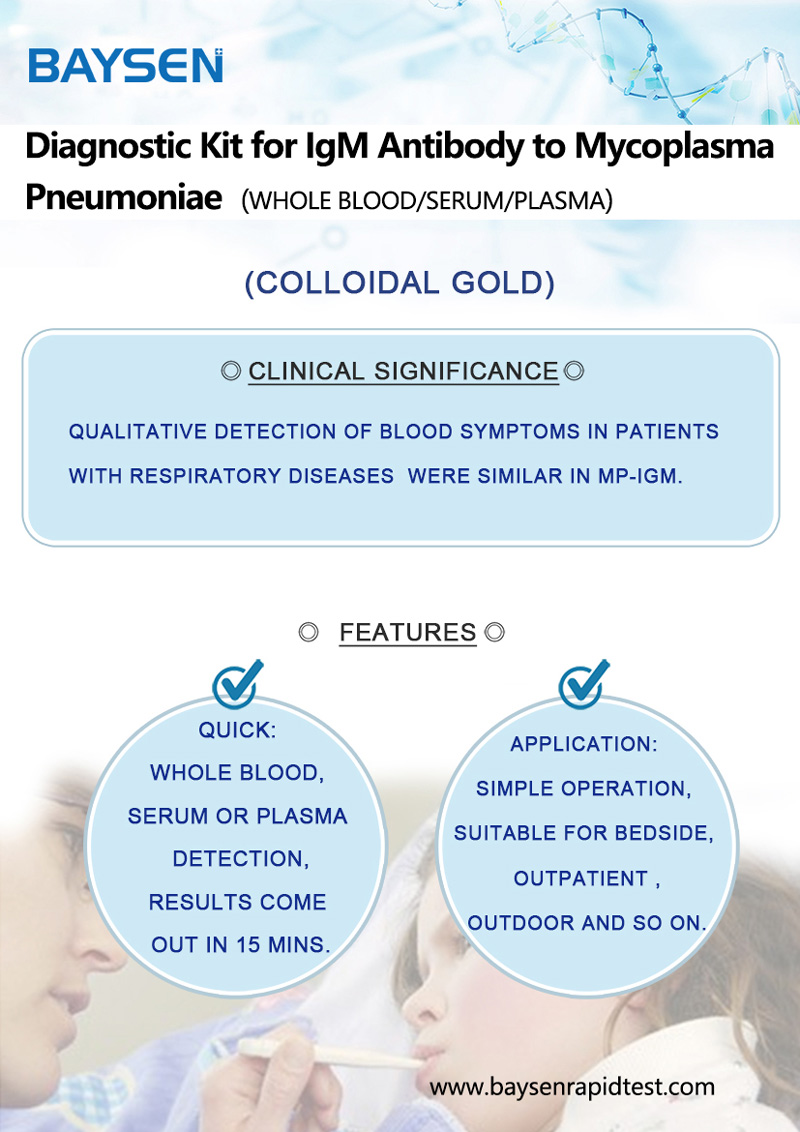
IgM antibody to mycoplasma pneumoniae test kit colloidal gold
Products Parameters
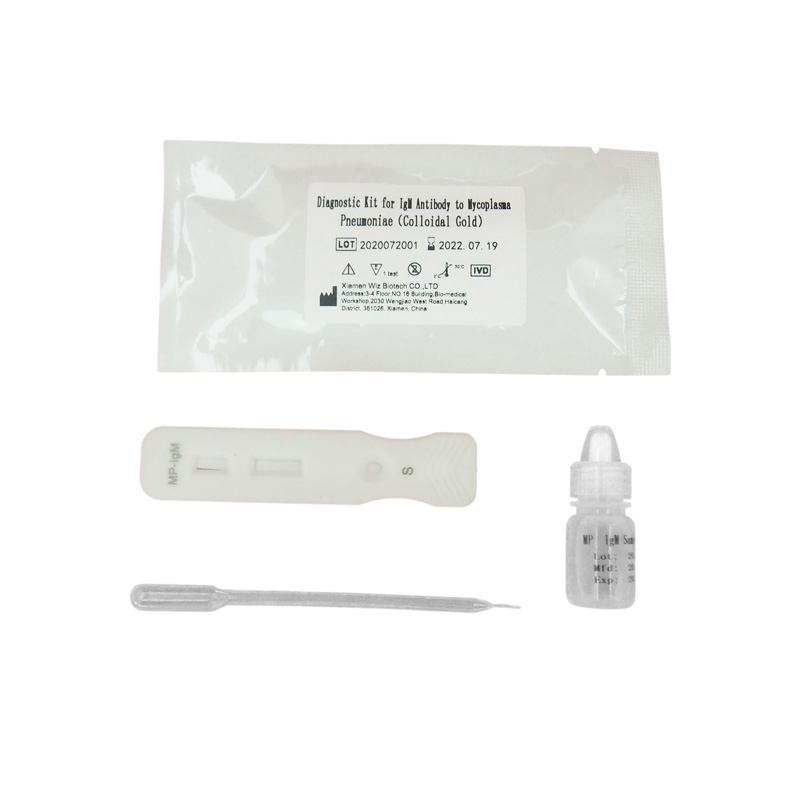
PRINCIPLE AND PROCEDURE OF FOB TEST
PRINCIPLE
The strip has MP-Ag coating antigen on test region and goat anti mouse IgG antibody on control region, which is fastened to membrane chromatography in advance. Lable pad is coated by colloidal gold labeled mouse-anti human IgM McAb in advance. When testing positive sample, the MP-IgM in sample combine with colloidal gold labeled mouse-anti human IgM McAb, and form immune complex. Under the action of the immunochromatography, the complex and sample inside of the nitrocellulose membrane flow in the direction of absorbent paper, when the complex passed the test region, it combined with MP-Ag coating antigen, forming “MP-Ag coating antigen-MP-IgM-colloidal gold labeled mouse-anti human IgM McAb” complex, a colored test band appeared on test region. A negative sample does not produce a test band due to deficient immune complex. No matter MP-IgM is present in sample or not, there is a red stripe appear on quality control region, which is regarded as quality internal enterprise standards.
Test Procedure:
The WIZ-A101 test procedure see the instruction of portable immune analyzer. Visual test procedure is as follows:
1. Lay aside all reagents and samples to room temperature.
2. Take out the test card from the foil bag, put it on the level table and mark it.
3. Add 10μL serum or plasma sample or 20μL whole blood sample to sample well of the card with provided dispette, then add 100μL (about 2-3 drop) sample diluent, start timing.
4. Wait for a minimum 10-15 minutes and read the result, the result is invalid after 15 minutes.

About Us

Xiamen Baysen Medical Tech limited is a high biological enterprise which devotes itself to filed of fast diagnostic reagent and integrates research and development, production and sales into a whole. There are many advanced research staffs and sales managers in the company, all of them are have rich working experience in china and international biopharmaceutical enterprise.
Certificate display