Popular Design for Immunoassay Test Kits - Diagnostic Kit(Colloidal Gold)for Fecal Occult Blood – Baysen
Popular Design for Immunoassay Test Kits - Diagnostic Kit(Colloidal Gold)for Fecal Occult Blood – Baysen Detail:
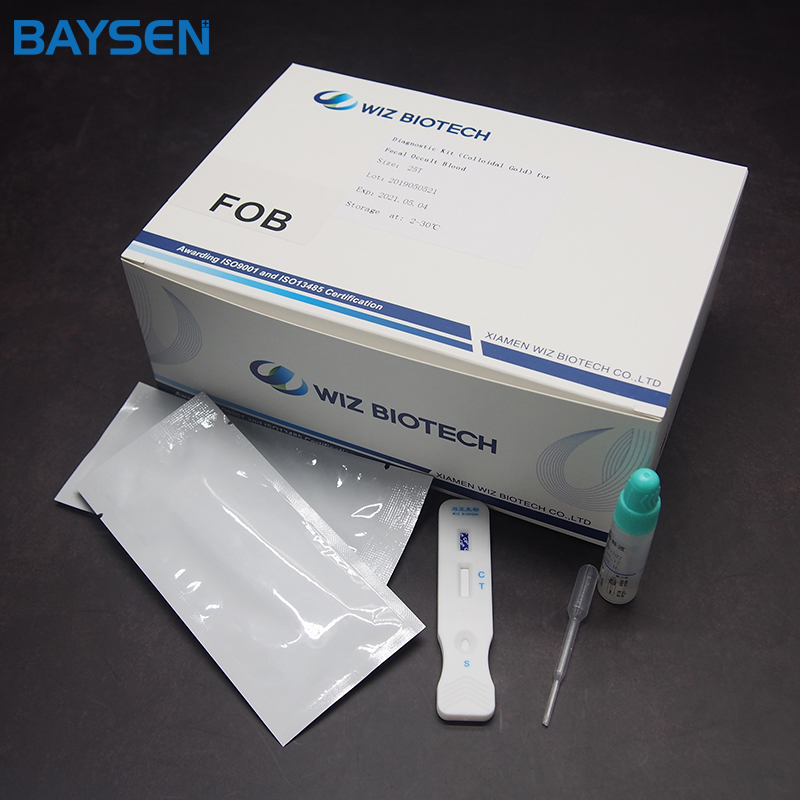
Diagnostic Kit(Colloidal Gold)for Fecal Occult Blood
For in vitro diagnostic use only
Please read this package insert carefully prior to use and strictly follow the instructions. Reliability of assay results cannot be guaranteed if there are any deviations from the instructions in this package insert.
INTENDED USE
Diagnostic Kit(Colloidal Gold)for Fecal Occult Blood (FOB) is a colloidal gold immunochromatographic assay for the qualitative determination of hemoglobin in human faeces, it acts as gastrointestinal bleeding auxiliary diagnosis reagent clinical diagnosis. This test is a screening reagent. All positive sample must be confirmed by other methodologies. This test is intended for healthcare professional use only. Meanwhile, this test is used for IVD, extra instruments are not needed.
PACKAGE SIZE
1 kit /box, 10 kits /box, 25 kits,/box, 100 kits /box
SUMMARY
The slight bleeding of digestive tract disease give rise to FOB, so the detection of FOB has important value for gastrointestinal bleeding disease auxiliary diagnosis, it is available approach for screening digestive tract diseases. The kit is a simple, visual qualitative test that detects hemoglobin in human faeces, it has high detection sensitivity and strong specificity. The test is based on immunochromatography and can give a result within 15 minutes.
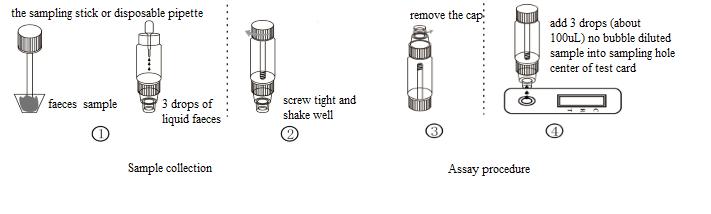
ASSAY PROCEDURE
1.Take out the sampling stick, inserted into the faeces sample, then put the sampling stick back, screw tight and shake well, repeat the action 3 times. Or using the sampling stick picked about 50mg faeces sample, and put in a faeces sample tube containing sample dilution, and screw tightly.
2.Use disposable pipette sampling take the thinner faeces sample from the diarrhea patient, then add 3 drops (about 100uL) to the fecal sampling tube and shake well, put aside.
3.Take out the test card from the foil bag, put it on the level table and mark it.
4Remove the cap from the sample tube and discard the first two drops diluted sample, add 3 drops (about 100uL) no bubble diluted sample verticaly and slowly into sample well of the card with provided dispette, start timing.
5.For the test strip: take out the test strip from the foil bag, put it on the level table and mark it. Dip the end with arrow of the strip into the sample solution, start timing.
6.The result should be read within 10-15 minutes, and it is invalid after 15 minutes.
Product detail pictures:


Related Product Guide:
Precision-Medicine Approach Could Revive Prostate Cancer Test | Calprotectin Elisa Kit
Is genetic testing sophisticated enough to make PSA screening viable for mainstream use? | P24 Test Strips
Our business has been focusing on brand strategy. Customers' pleasure is our best advertising. We also offer OEM company for Popular Design for Immunoassay Test Kits - Diagnostic Kit(Colloidal Gold)for Fecal Occult Blood – Baysen , The product will supply to all over the world, such as: Puerto Rico, Bahrain, Denmark, By integrating manufacturing with foreign trade sectors, we can offer total customer solutions by guaranteeing the delivery of right items to the right place at the right time, which is supported by our abundant experiences, powerful production capability, consistent quality, diversified product portfolios and the control of the industry trend as well as our mature before and after sales services. We'd like to share our ideas with you and welcome your comments and questions.
Timely delivery, strict implementation of the contract provisions of the goods, encountered special circumstances, but also actively cooperate, a trustworthy company!