OEM/ODM Supplier Infectious Disease Rapid Test Kits - Diagnostic kit for Microalbuminuria (Alb) – Baysen
OEM/ODM Supplier Infectious Disease Rapid Test Kits - Diagnostic kit for Microalbuminuria (Alb) – Baysen Detail:
Diagnostic Kit for Urine microalbumin
(Fluorescence Immunochromatographic Assay)
For in vitro diagnostic use only
Please read this package insert carefully prior to use and strictly follow the instructions. Reliability of assay results cannot be guaranteed if there are any deviations from the instructions in this package insert.
INTENDED USE
Diagnostic Kit for Urine microalbumin (Fluorescence Immunochromatographic Assay) is suitable for the quantitative detection of microalbumin in human urine by fluorescence immunochromatographic assay, which is mainly used for the auxiliary diagnosis of kidney disease.All positive sample must be confirmed by other methodologies. This test is intended for healthcare professional use only.
SUMMARY
Microalbumin is a normal protein found in the blood and is extremely rare in urine when metabolized normally. If there’s a trace amount in the urine Albumin in more than 20 micron /mL, belongs to urinary microalbumin, if can be timely treatment, can completely repair the glomeruli, eliminate proteinuria, if not timely treatment, may enter the uremia phase.The increase of urinary microalbumin is mainly seen in diabetic nephropathy, hypertension and preeclampsia in pregnancy. The condition can be accurately diagnosed by the value of urinary microalbumin, combined with the incidence, symptoms and medical history. Early detection of urinary microalbumin is very important to prevent and delay the development of diabetic nephropathy.
PRINCIPLE OF THE PROCEDURE
The membrane of the test device is coated with ALB antigen on the test region and goat anti rabbit IgG antibody on the control region. Marker pad are coated by fluorescence mark anti ALB antibody and rabbit IgG in advance. When testing sample, ALB in sample combine with fluorescence marked anti ALB antibody, and form immune mixture. Under the action of the immunochromatography, the complex flow in the direction of absorbent paper, when complex passed the test region, The free fluorescent marker will be combined with ALB on the membrane.The concentration of ALB is negative correlation for fluorescence signal, and the concentration of ALB in sample can be detected by fluorescence immunoassay assay.
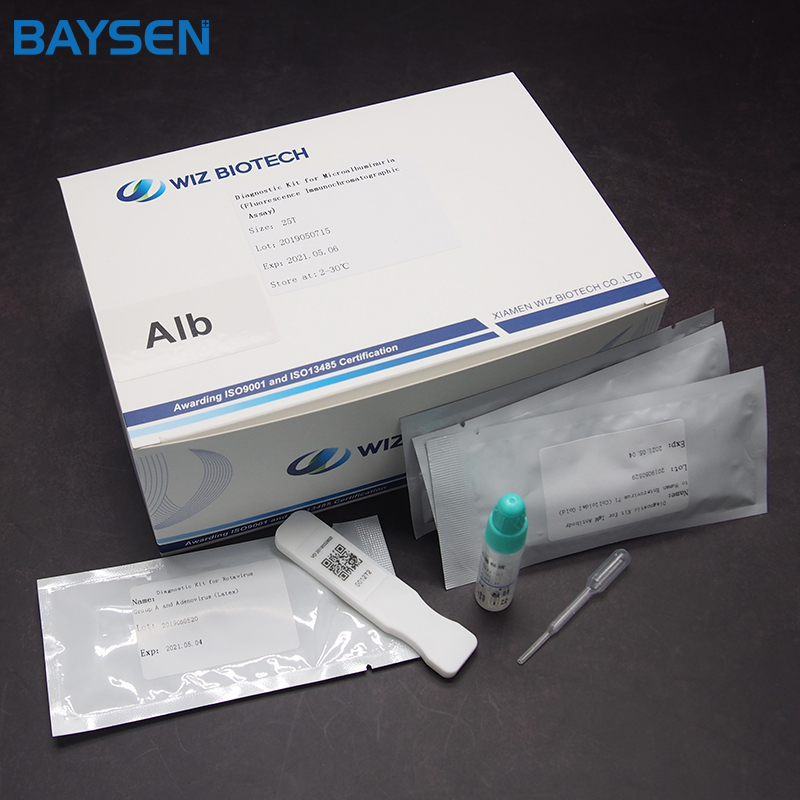
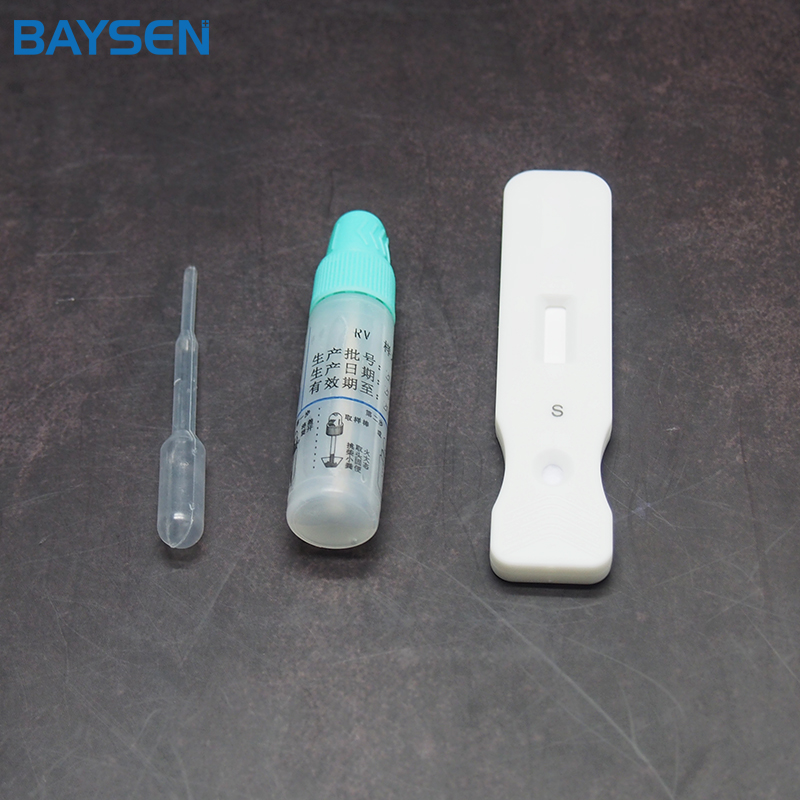
REAGENTS AND MATERIALS SUPPLIED
25T package components:
Test card individually foil pouched with a desiccant 25T
Package insert 1
MATERIALS REQUIRED BUT NOT PROVIDED
Sample collection container,timer
SAMPLE COLLECTION AND STORAGE
- The samples tested can be urine.
- Fresh urine samples can be collected in a disposable clean container. It is recommended to test the urine samples immediately after collection. If the urine samples cannot be tested immediately, please store them at 2-8 ℃, but it is recommended not to store them for more than 12 hours. Do not shake the container. If there is sediment at the bottom of the container, take supernatant for testing.
- All sample avoid freeze-thaw cycles.
- Thaw samples to room temperature before use.
Product detail pictures:

Related Product Guide:
The use of Piper sarmentosum leaves aqueous extract (Kadukmy™) as antihypertensive agent in spontaneous hypertensive rats | BMC Complementary and Alternative Medicine | P24 Test Strips
Multiplexed and portable nucleic acid detection platform with Cas13, Cas12a, and Csm6 | Diagnostic Kit For Isoenzyme Mb Of C Reatine Kinase
With this motto in mind, we've turn into one of quite possibly the most technologically innovative, cost-efficient, and price-competitive manufacturers for OEM/ODM Supplier Infectious Disease Rapid Test Kits - Diagnostic kit for Microalbuminuria (Alb) – Baysen , The product will supply to all over the world, such as: Brisbane, Oslo, United Arab Emirates, With a wide range, good quality, reasonable prices and stylish designs, our items are extensively used in this field and other industries. We welcome new and old customers from all walks of life to contact us for future business relationships and achieving mutual success! We welcome customers, business associations and friends from all parts of the world to contact us and seek cooperation for mutual benefits.
It is not easy to find such a professional and responsible provider in today's time. Hope that we can maintain long-term cooperation.












