OEM/ODM Factory Elisa Kit - Pepsinogen I Pepsinogen II and Gastrin-17 Combo rapid test kit – Baysen
OEM/ODM Factory Elisa Kit - Pepsinogen I Pepsinogen II and Gastrin-17 Combo rapid test kit – Baysen Detail:
Diagnostic Kit for Pepsinogen I/Pepsinogen II /Gastrin-17
Methodology:fluorescence immunochromatographic assay
Production information
| Model Number | G17/PGI/PGII | Packing | 25 Tests/ kit, 30kits/CTN |
| Name | Diagnostic Kit for Pepsinogen I/Pepsinogen II /Gastrin-17 | Instrument classification | Class II |
| Features | High sensitivity, Easy opeation | Certificate | CE/ ISO13485 |
| Accuracy | > 99% | Shelf life | Two Years |
| Methodology | fluorescence immunochromatographic assay | OEM/ODM service | Avaliable |
INTENDED USE
This kit is applicable to the in vitro quantitative detection of concentration of Pepsinogen I (PGI), Pepsinogen II
(PGII) and Gastrin 17 in human serum/plasma/whole blood samples, to evaluate gastric oxyntic gland cell
function, gastric fundus mucosa lesion and atrophic gastritis. The kit only provides test result of Pepsinogen I
(PGI), Pepsinogen II (PGII) and Gastrin 17. The obtained result shall be analyzed in combination with other clinical
information. It must only be used by healthcare professionals.
Test procedure
| 1 | Before using the reagent,read the package insert carefully and familiarize yourself with the operating procedures. |
| 2 | Select standard test mode of WIZ-A101 portable immune analyzer. |
| 3 | Open the aluminum foil bag package of reagent and take out the test device. |
| 4 | Horizontally insert the test device into the slot of immune analyzer. |
| 5 | On home page of operation interface of immune analyzer, click “Standard” to enter test interface |
| 6 | Click “QC Scan” to scan the QR code on inner side of the kit; input kit related parameters into instrument and select sample type. Note: Each batch number of the kit shall be scanned for one time. If the batch number has been scanned, then skip this step. |
| 7 | Check the consistency of “Product Name”, “Batch Number” etc. On test interface with information on the kit label. |
| 8 | After information consistency is confirmed, take out sample diluents, add 80µL of serum/plasma/whole blood sample, and sufficiently mix. |
| 9 | Add 80µL of above mixed solution into the sample hole of test device. |
| 10 | After complete sample addition, click “Timing” and remaining test time will be automatically displayed on the interface. |
| 11 | Immune analyzer will automatically complete test and analysis when test time is reached. |
| 12 | Result calculation and display After test by immune analyzer is completed, test result will be displayed on test interface or can be viewed through “History” on home page of operation interface. |
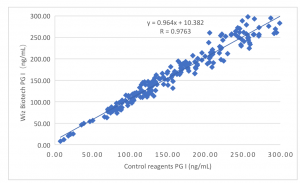
The Clinical Performance
Clinical evaluation performance of the product is assessed by collecting 200 clinical samples. Use the marketed kit of enzyme linked immunosorbent assay as the control reagent. Compare the PGI test results. Use linearity regression to investigate their comparability. Correlation coefficients of two tests are y = 0.964X + 10.382 and R=0.9763 respectively. Compare the PGII test results. Use linearity regression to investigate their comparability. Correlation coefficients of two tests are y = 1.002X + 0.025 and R=0.9848 respectively. Compare the G-17 test results. Use linearity regression to investigate their comparability. Correlation coefficients of two tests are y =0.983X + 0.079 and R=0.9864 respectively.
Product detail pictures:



Related Product Guide:
MDxHealth (R): SelectMDx Cost-effective in Four European Countries Brussels Stock Exchange:MDXH | Calprotectin Elisa Kit
Most Men Don’t Need a PSA Test for Prostate Cancer | Cpn-Igm
So as to provide you with ease and enlarge our business, we even have inspectors in QC Crew and guarantee you our best company and solution for OEM/ODM Factory Elisa Kit - Pepsinogen I Pepsinogen II and Gastrin-17 Combo rapid test kit – Baysen , The product will supply to all over the world, such as: Portugal, Uruguay, New Zealand, Over the years, with high-quality products, first- class service, ultra-low prices we win thee trust and favor of customers. Nowadays our products sell all over the domestic and abroad. Thanks for the regular and new customers support. We provide high quality product and competitive price, welcome the regular and new customers cooperate with us!
Good quality, reasonable prices, rich variety and perfect after-sales service, it's nice!