OEM/ODM Factory Elisa Kit - Diabetes management Insulin Diagnostic kit – Baysen
OEM/ODM Factory Elisa Kit - Diabetes management Insulin Diagnostic kit – Baysen Detail:
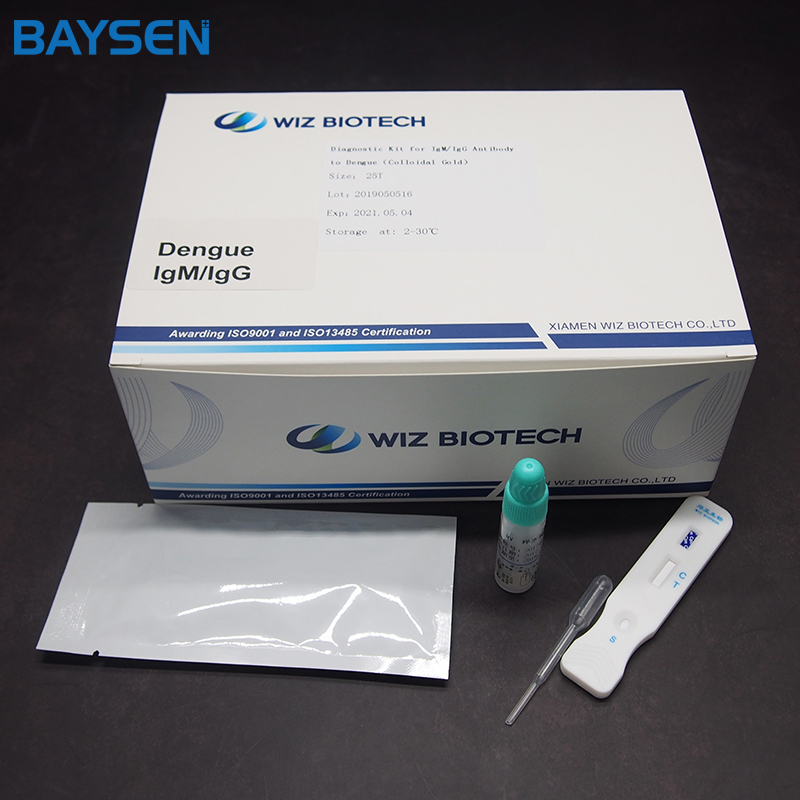
Diagnostic Kit for Insulin
Methodology:Fluorescence Immunochromatographic Assay
Production information
| Model Number | INS | Packing | 25 Tests/ kit, 30kits/CTN |
| Name | Diagnostic Kit for Insulin | Instrument classification | Class Ii |
| Features | High sensitivity, Easy opeation | Certificate | CE/ ISO13485 |
| Accuracy | > 99% | Shelf life | Two Years |
| Methodology | Fluorescence Immunochromatographic Assay | OEM/ODM service | Avaliable |

INTENDED USE
This kit is suitable for the in vitro quantitative determination of insulin (INS) levels in human serum/plasma/whole blood samples for the evaluation of pancreatic-islet β-cell function. This kit only provides insulin (INS) test results, and the obtained result shall be analyzed in combination with other clinical information. result shall be analyzed in combination with other clinical information.
Test procedure
| 1 | Before using the reagent,read the package insert carefully and familiarize yourself with the operating procedures. |
| 2 | Select standard test mode of WIZ-A101 portable immune analyzer |
| 3 | Open the aluminum foil bag package of reagent and take out the test device. |
| 4 | Horizontally insert the test device into the slot of immune analyzer. |
| 5 | On home page of operation interface of immune analyzer, click “Standard” to enter test interface. |
| 6 | Click “QC Scan” to scan the QR code on inner side of the kit; input kit related parameters into instrument and select sample type. Note: Each batch number of the kit shall be scanned for one time. If the batch number has been scanned, then skip this step. |
| 7 | Check the consistency of “Product Name”, “Batch Number” etc. on test interface with information on the kit label. |
| 8 | Take out sample diluent upon consistent information, add 10μL serum/plasma/whole blood sample, and thoroughly mix them; |
| 9 | Add 80µL aforesaid thoroughly mixed solution into well of test device; |
| 10 | After complete sample addition, click “Timing” and remaining test time will be automatically displayed on the interface. |
| 11 | Immune analyzer will automatically complete test and analysis when test time is reached. |
| 12 | After test by immune analyzer is completed, test result will be displayed on test interface or can be viewedthrough “History” on home page of operation interface. |
Note: each sample shall be pipetted by clean disposable pipette to avoid cross contamination.
Clinical Performance
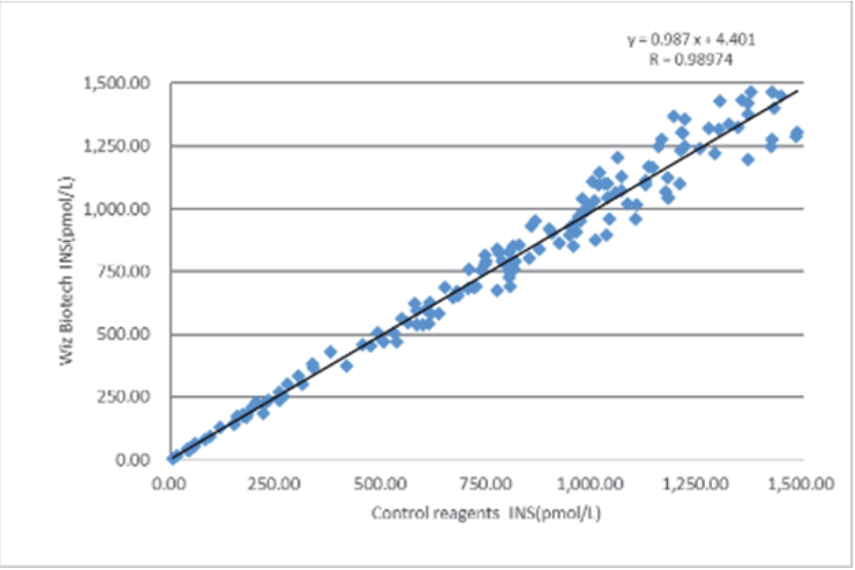
The clinical evaluation performance of this product was evaluated by collecting 173 clinical samples. The results of the tests were compared using the corresponding kits of the marketed electrochemiluminescence method as reference reagents, and their comparability was investigated by linear regression, and the correlation coefficients of the two tests were y = 0.987x+4.401 and R = 0.9874, respectively.
Product detail pictures:



Related Product Guide:
Why Dutch hospitals are so good at beating superbugs | Cpn-Igm
Cleveland Diagnostics Study Finds Its IsoPSA Test Outperforms Standard PSA Testing | P24 Test Strips
Dedicated to strict quality control and thoughtful customer service, our experienced staff members are always available to discuss your requirements and ensure full customer satisfaction for OEM/ODM Factory Elisa Kit - Diabetes management Insulin Diagnostic kit – Baysen , The product will supply to all over the world, such as: belarus, Mexico, Saudi Arabia, Our company absorbs new ideas, strict quality control, a full range of service tracking, and adhere to make high-quality solutions. Our business aims to "honest and trustworthy, favorable price, customer first", so we won the trust of the majority of customers! If you are interested in our items and services, please do not hesitate to contact us!
This is a very professional and honest Chinese supplier, from now on we fell in love with the Chinese manufacturing.