OEM/ODM China Cea Carcinoembryonic Antigen Test - Diagnostic Kit(Colloidal Gold)for Follicle-stimulating Hormone – Baysen
OEM/ODM China Cea Carcinoembryonic Antigen Test - Diagnostic Kit(Colloidal Gold)for Follicle-stimulating Hormone – Baysen Detail:
Diagnostic Kit(Colloidal Gold)for Follicle-stimulating Hormone
For in vitro diagnostic use only
Please read this package insert carefully prior to use and strictly follow the instructions. Reliability of assay results cannot be guaranteed if there are any deviations from the instructions in this package insert.
INTENDED USE
The kit is used for qualitative detection of follicle-stimulating hormone (FSH) levels in urine samples. It is suitable for assisting determination the appearance of female menopause.
PACKAGE SIZE
1 kit /box, 10 kits /box, 25 kits,/box, 50 kits /box.
SUMMARY
FSH is a glycoprotein hormone secreted by the pituitary gland, it can enter blood and urine through blood circulation. For male, FSH promotes maturity of testicular seminiferous tubule and the production of sperm, for female, FSH promotes follicular development and maturity, and collaborates LH to mature follicles secrete estrogen and ovulation, involved in the formation of normal menstruation[1]. FSH maintains a consistently stable basal level in normal subjects, about 5-20mIU/mL. Female menopause typically occurs between the ages of 49 and 54, and lasts for an average of four to five years. During this period, because of ovarian atrophy, follicular atresia and degeneration, estrogen secretion decreased significantly, a large number of stimulating pituitary gonadotropin secretion, especially FSH levels will be significantly increased, is generally 40-200mIU/ml, and maintain the level in a very long time[2]. This kit based on colloidal gold immune chromatography analysis technology for qualitative detection of FSH antigen in human urine samples, which can give a result within 15 minutes.
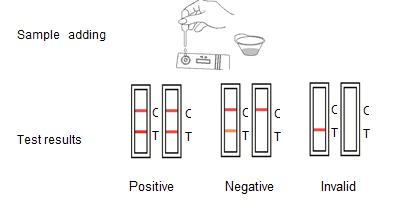
ASSAY PROCEDURE
1.Take out the test card from the foil bag, put it on the level table and mark it.
2.Discard the first two drops sample, add 3 drops (about 100μL) no bubble sample verticaly and slowly into sample well of the card with provided dispette, start timing.
3.The result should be read within 10-15 minutes, and it is invalid after 15 minutes.
Product detail pictures:

Related Product Guide:
SIU 2018: Biomarkers Are Cost Effective and Useful in Patient Selection | Psa Test Cost
Weekly Dose: Keytruda may be a miracle cancer drug, but can those who need it afford it? | Psa Test Cost
Innovation, good quality and reliability are the core values of our enterprise. These principles today extra than ever form the basis of our success as an internationally active mid-size organization for OEM/ODM China Cea Carcinoembryonic Antigen Test - Diagnostic Kit(Colloidal Gold)for Follicle-stimulating Hormone – Baysen , The product will supply to all over the world, such as: Eindhoven, Belize, South Africa, We care about every steps of our services, from factory selection, product development & design, price negotiation, inspection, shipping to aftermarket. Now we have implemented a strict and complete quality control system, which ensures that each product can meet quality requirements of customers. Besides, all of our solutions have been strictly inspected before shipment. Your Success, Our Glory: Our aim is to help customers realize their goals. We're making great efforts to achieve this win-win situation and sincerely welcome you to join us.
The sales manager is very patient, we communicated about three days before we decided to cooperate, finally, we are very satisfied with this cooperation!