OEM Manufacturer Hiv 1+2 Test Kit Whole Blood/serum/plasma - home test one step Rotavirus Group A test kit latex RV test IVD reagent – Baysen
OEM Manufacturer Hiv 1+2 Test Kit Whole Blood/serum/plasma - home test one step Rotavirus Group A test kit latex RV test IVD reagent – Baysen Detail:
Products Parameters
PRINCIPLE AND PROCEDURE OF FOB TEST
PRINCIPLE
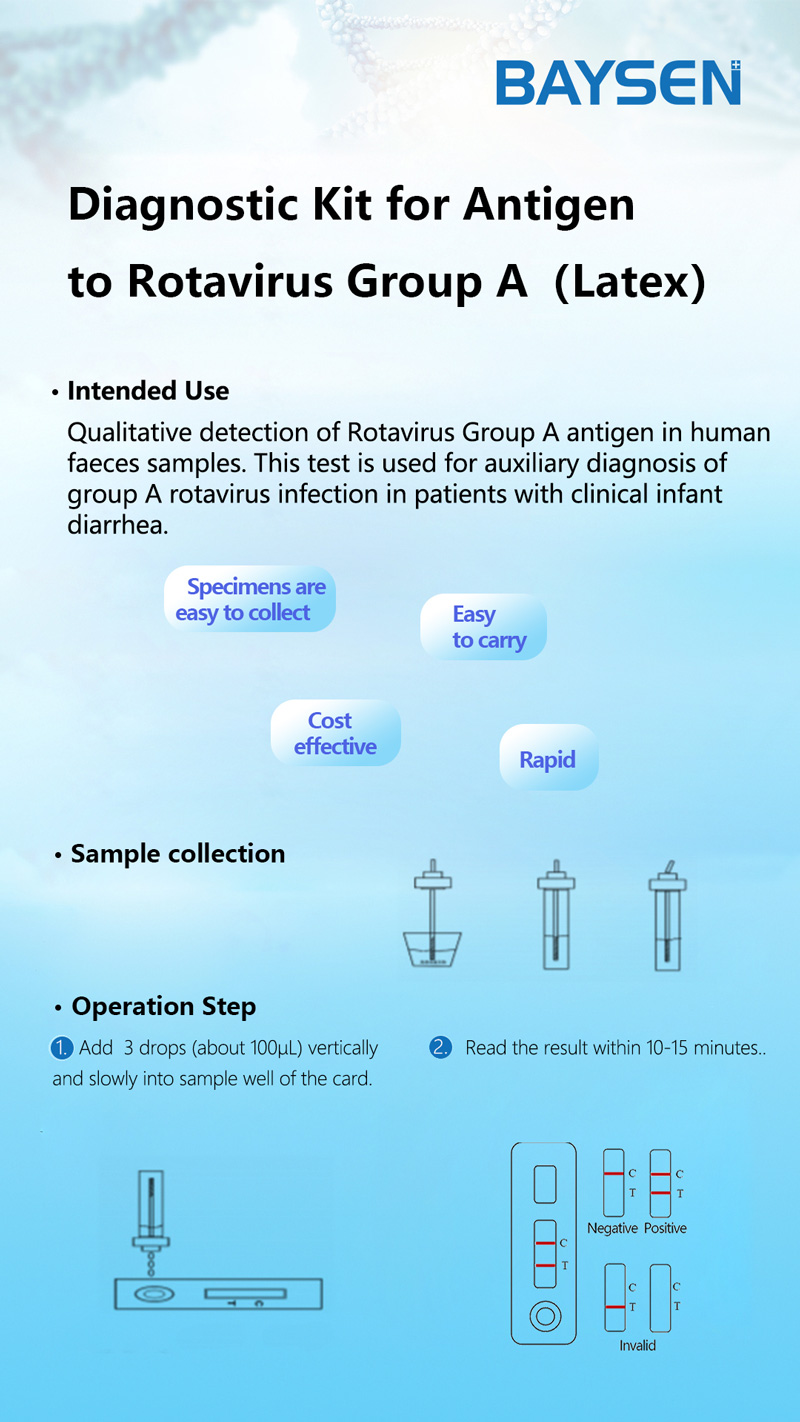
The membrane of the test device is coated with Rotavirus Group A antigen on the test region and goat anti rabbit IgG antibody on the control region. Lable pad are coated by fluorescence labeled anti Rotavirus Group A and rabbit IgG in advance. When testing positive sample, the RV in sample combine with fluorescence labeled anti Rotavirus Group A, and form immune mixture. Under the action of the immunochromatography, the complex flow in the direction of absorbent paper. When complex passed the test region, it combined with anti-Rotavirus Group A coating antibody, forms new complex. If it is negative, there is no Rotavirus Group A antigen in the sample, so that immune complexes cannot be formed, there will be no red line in the detection area (T). Regardless of whether group A rotavirus is present in the specimen, the latex-labeled mouse IgG is chromatographed to the quality control area (C) and captured by goat anti-mouse IgG antibody. A red line will appear in the quality control area (C). The red line is the standard appears in the quality control area (C) for judging whether there are enough samples and whether the chromatography process is normal. It is also used as an internal control standard for reagents.
Test Procedure:
1.Symptomatic patients should be collected. According to reports, the maximum excretion of rotavirus in the faeces of patients with gastroenteritis occurs 3-5 days after the begin of the disease and 3-13 days after the start of symptoms. If the sample is collected long after the diarrhea, the number of antigens may not be enough to occur the positive reaction.
2.The samples should be collected in a clean, dry, waterproof container which does not contain detergents and preservatives.
3.For non-diarrhea patients, the collected faeces samples should not be less than 1-2 grams. For patients with diarrhea, if the faeces is liquid, please collect at least 1-2 ml of faeces liquid. If the faeces contains a lot of blood and mucus, please collect the sample again.
4.It is recommended to test the samples immediately after collection, otherwise they should be sent to the laboratory within 6 hours and stored at 2-8°C. If the samples have not been tested within 72 hours, they should be stored at the temperature below -15°C.
5.Use fresh feces for testing, and faeces samples mixed with diluent or distilled water

You May like
SARS-CoV-2 Antigen Rapid Test(Colloidal Gold)
WIZ-A101 Portable Immune Analyzer
Diagnostic Kit for Helicobacter pylori Antibody (Colloidal Gold)
About Us

Xiamen Baysen Medical Tech limited is a high biological enterprise which devotes itself to filed of fast diagnostic reagent and integrates research and development, production and sales into a whole. There are many advanced research staffs and sales managers in the company, all of them are have rich working experience in china and international biopharmaceutical enterprise.
Certificate display

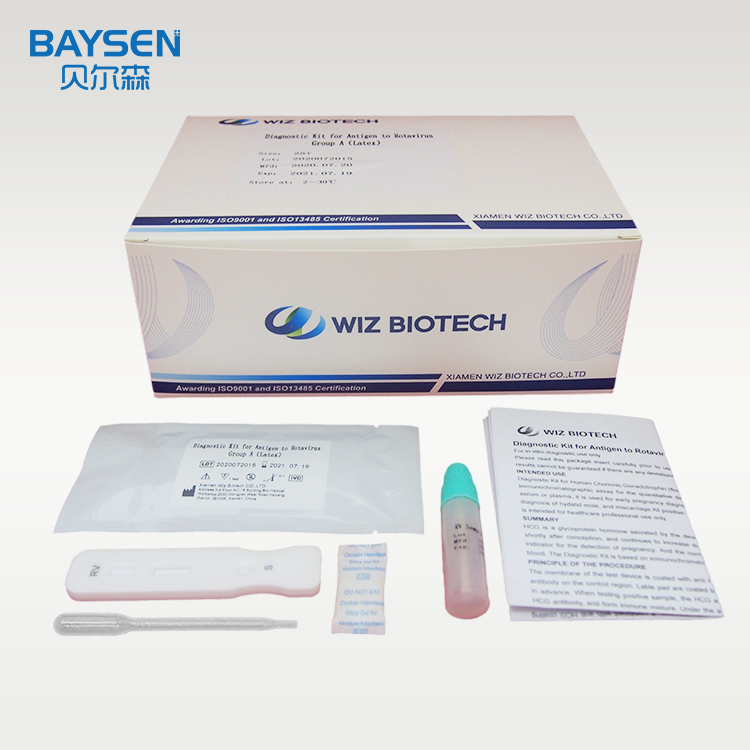
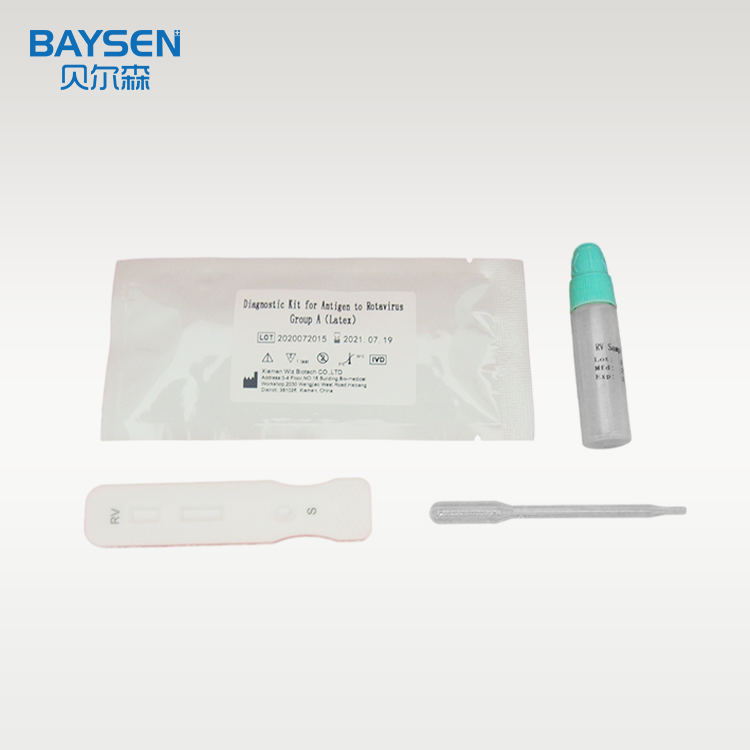
Product detail pictures:



Related Product Guide:
Guys: You Don’t Want That PSA Test For Prostate Cancer | Psa Test Cost
Myeloid-related protein 8 induces self-tolerance and cross-tolerance to bacterial infection via TLR4- and TLR2-mediated signal pathways | Cpn-Igm
Our merchandise are commonly recognized and reliable by customers and can meet constantly developing economic and social desires for OEM Manufacturer Hiv 1+2 Test Kit Whole Blood/serum/plasma - home test one step Rotavirus Group A test kit latex RV test IVD reagent – Baysen , The product will supply to all over the world, such as: London, Muscat, Roman, Please really feel free to send us your requirements and we'll respond to you asap. We have got a professional engineering group to serve for your just about every detailed needs. Cost-free samples could be sent for you personally to understand much more information. In an effort to meet your requires, please really feel free to make contact with us. You may send us emails and contact us directly. Moreover, we welcome visits to our factory from around the globe for much better recognizing of our organization. nd items. In our trade with merchants of numerous countries, we usually adhere for the principle of equality and mutual benefit. It is actually our hope to market, by joint efforts, each trade and friendship to our mutual advantage. We look forward to getting your inquiries.
The sales manager has a good English level and skilled professional knowledge, we have a good communication. He is a warm and cheerful man, we have a pleasant cooperation and we became very good friends in private.