New Fashion Design for Baby Dining Table Set - 8 Years Exporter China Accurate HCV Rapid Diagnostic Test Rapid Test Kits in Pathological Analysis Equipments – Baysen
New Fashion Design for Baby Dining Table Set - 8 Years Exporter China Accurate HCV Rapid Diagnostic Test Rapid Test Kits in Pathological Analysis Equipments – Baysen Detail:
We enjoy an extremely good status among our prospects for our great merchandise top quality, competitive price and the ideal service for 8 Years Exporter China Accurate HCV Rapid Diagnostic Test Rapid Test Kits in Pathological Analysis Equipments, We’ve been self-confident that there will be considered a promising upcoming and we hope we could have long term cooperation with prospects from all over the environment.
We enjoy an extremely good status among our prospects for our great merchandise top quality, competitive price and the ideal service for China Rapid Test, HCV Rapid Diagnostic Test, Our goods are very popular in the word, like South American, Africa, Asia and so on. Companies to “create first-class products” as the goal, and strive to offer customers with high quality solutions, supply high-quality after-sales service and technical support, and customer mutual benefit, create a better career and future!
For in vitro diagnostic use only
Please read this package insert carefully prior to use and strictly follow the instructions. Reliability of assay results cannot be guaranteed if there are any deviations from the instructions in this package insert.
INTENDED USE
Diagnostic Kit for Hepatitis C Virus Antibody (Fluorescence Immunochromatographic Assay)is a fluorescence immunochromatographic assay for the quantitative detection of HCV antibody in human serum or plasma,which is important auxiliary diagnostic value for infection with hepatitis C.All positive sample must be confirmed by other methodologies. This test is intended for healthcare professional use only
1.Lay aside all reagents and samples to room temperature.
2.Open the Portable Immune Analyzer(WIZ-A101), enter the account password login according to the operation method of the instrument, and enter the detection interface.
3.Scan the dentification code to confirm the test item.
4.Take out the test card from the foil bag.
5.Insert the test card into the card slot, scan the QR code, and determine the test item.
6.Add 20μL serum or plasma sample to sample diluent, and mix well..
7.Add 80μL sample solution to sample well of the card.
8.Click the “standard test” button, after 15 minutes, the instrument will automatically detect the test card, it can read the results from the display screen of the instrument, and record/print the test results.
9.Refer to the instruction of Portable Immune Analyzer(WIZ-A101).
SUMMARY
Hepatitis C virus (HCV) is an envelope, single stranded positive sense RNA (9.5 kb) virus belonging to the family of Flaviviridae. Six major genotypes and series of subtypes of HCV have been identified. Isolated in 1989, HCV is now recognized as the major cause for transfusion associated non-A, non-B hepatitis. The disease is characterized with acute and chronic form. More than 50% of the infected individuals develop severe, life threatening chronic hepatitis with liver cirrhosis and hepatocellular carcinomas. Since the introduction in 1990 of anti-HCV screening of blood donations, the incidence of this infection in transfusion recipients has been significantly reduced. Clinical studies show that significant amount of HCV infected individuals develop antibodies to NS5 non-structural protein of the virus. For this, the tests include antigens from the NS5 region of the viral genome in addition to NS3 (c200), NS4 (c200) and the Core (c22).
PRINCIPLE OF THE PROCEDURE
The membrane of the test device is coated with HCV antigen on the test region and goat anti rabbit IgG antibody on the control region. Lable pad are coated by fluorescence labeled HCV antigen and rabbit IgG in advance. When testing positive sample, the HCV antibody in sample combine with fluorescence labeled HCV antigen, and form immune mixture. Under the action of the immunochromatography, the complex flow in the direction of absorbent paper, when complex passed the test region, it combined with HCV antigen coating antigen, forms new complex.HCV antibody level is positively correlated with fluorescence signal, and the concentration of HCV antibody in sample can be detected by fluorescence immunoassay assay
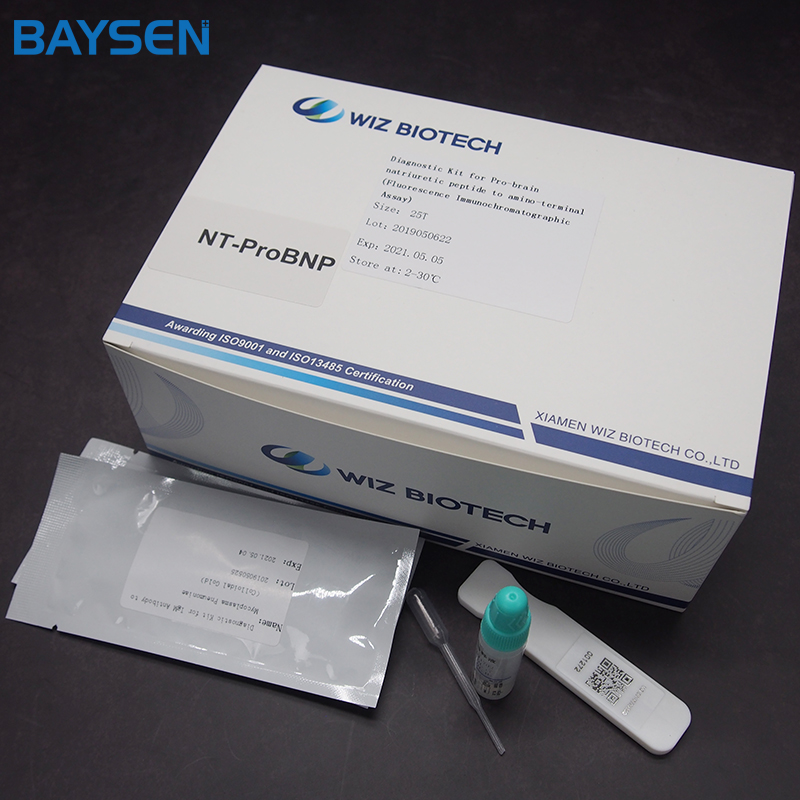
REAGENTS AND MATERIALS SUPPLIED
25T package components:
.Test card individually foil pouched with a desiccant
.Sample diluents
.Package insert
MATERIALS REQUIRED BUT NOT PROVIDED
Sample collection container,timer
SAMPLE COLLECTION AND STORAGE
1.The samples tested can be serum, heparin anticoagulant plasma or EDTA anticoagulant plasma.
2.According to standard techniques collect sample. Serum or plasma sample can be kept refrigerated at 2-8℃ for 7days and cryopreservation below -15°C for 6 months
3.All sample avoid freeze-thaw cycles.
ASSAY PROCEDURE
Please read the instrument operation manual and package insert before testing.
.This test result is only for clinical reference, should not serve as the only basis for clinical diagnosis and treatment, the patients clinical management should be comprehensive consideration combined with its symptoms, medical history, other laboratory examination, treatment response, epidemiology and other information.
.This reagent is only used for serum and plasma tests. It may not obtain accurate result when used for other samples such as saliva and urine and etc.
PERFORMANCE CHARACTERISTICS
| Linearity | 0.005-5 | relative deviation:-15% to +15%. |
| Linear correlation coefficient:(r)≥0.9900 | ||
| Accuracy | The recovery rate shall be within 85% – 115%. | |
| Repeatability | CV≤15% | |
REFERENCES
1.Post transfusion hepatitis. In: Moore SB, ed. Transfusion-Transmitted Viral Diseases. Alington, VA. Am. Assoc. Blood Banks, pp. 53-38.
2.Hansen JH,et al.HAMA Interference with Murine Monoclonal Antibody-Based Immunoassays[J].J of Clin Immunoassay,1993,16:294-299.
3.Levinson SS.The Nature of Heterophilic Antibodies and the Role in Immunoassay Interference[J].J of Clin Immunoassay,1992,15:108-114.
4.Alter HJ., Purcell RH, Holland PV, et al. (1978) Transmissible agent in non-A, non-B hepatitis. Lancet I: 459-463.
5.Choo Q-L,Weiner AJ, Overby LR, Kuo G, Houghton M. (1990) Hepatitis C Virus: the major causative agent of viral non-A, non-B hepatitis. Br Med Bull 46: 423-441.
6.Engvall E, Perlmann P. (1971) Enzyme linked immunosorbent assay (ELISA):qualitative assay of IgG. Immunochemistry 8:871-874.
EXPECTED VALUES
HCV-Ab<0.02
It is recommended that each laboratory establish its own normal range representing its patient population.
TEST RESULTS AND INTERPRETATION
- The above data is the result of HCV-Ab reagent test, and it is suggested that each laboratory should establish a range of HCV-Ab detection values suitable for the population in this region. The above results are for reference only.
- The results of this method are only applicable to the reference ranges established in this method, and there is no direct comparability with other methods.
- Other factors can also cause errors in detection results, including technical reasons, operational errors and other sample factors.
STORAGE AND STABILITY
- The kit is 18 months shelf-life from the date of manufacture. Store the unused kits at 2-30°C. DO NOT FREEZE. Do not use beyond the expiration date.
- Do not open the sealed pouch until you are ready to perform a test, and the single-use test is suggested to be used under the required environment (temperature 2-35℃, humidity 40-90%) within 60 mins as quickly as possible.
- Sample diluent is used immediately after being opened.
WARNINGS AND PRECAUTIONS
.The kit should be sealed and protected against moisture.
.All positive specimens shall be validated by other methodologies.
.All specimens shall be treated as potential pollutant.
.DO NOT use expired reagent.
.DO NOT interchange reagents among kits with different lot No..
.DO NOT reuse test cards and any disposable accessories.
.Misoperation, excessive or little sample can lead to result deviations.
LIMITATION
.As with any assay employing mouse antibodies, the possibility exists for interference by human anti-mouse antibodies (HAMA) in the specimen. Specimens from patients who have received preparations of monoclonal antibodies for diagnosis or therapy may contain HAMA. Such specimens may cause false positive or false negative results.
Key to symbols used:
 |
In Vitro Diagnostic Medical Device |
 |
Manufacturer |
 |
Store at 2-30℃ |
 |
Expiration Date |
 |
Do Not Reuse |
 |
CAUTION |
 |
Consult Instructions For Use |
Product detail pictures:



Related Product Guide:
A New $500 Blood Test Could Detect Cancer Before Symptoms Develop | Diagnostic Kit For Isoenzyme Mb Of C Reatine Kinase
‘My life was probably saved by a £40 blood test for prostate cancer’ | Diagnostic Kit For Isoenzyme Mb Of C Reatine Kinase
We often persist with the theory "Quality To start with, Prestige Supreme". We are fully committed to delivering our clientele with competitively priced good quality items, prompt delivery and experienced support for New Fashion Design for Baby Dining Table Set - 8 Years Exporter China Accurate HCV Rapid Diagnostic Test Rapid Test Kits in Pathological Analysis Equipments – Baysen , The product will supply to all over the world, such as: Guyana, New Delhi, Canada, We focus on providing service for our clients as a key element in strengthening our long-term relationships. Our continual availability of high grade products in combination with our excellent pre-sale and after-sales service ensures strong competitiveness in an increasingly globalized market.
The quality of the products is very good, especially in the details, can be seen that the company work actively to satisfy customer's interest, a nice supplier.