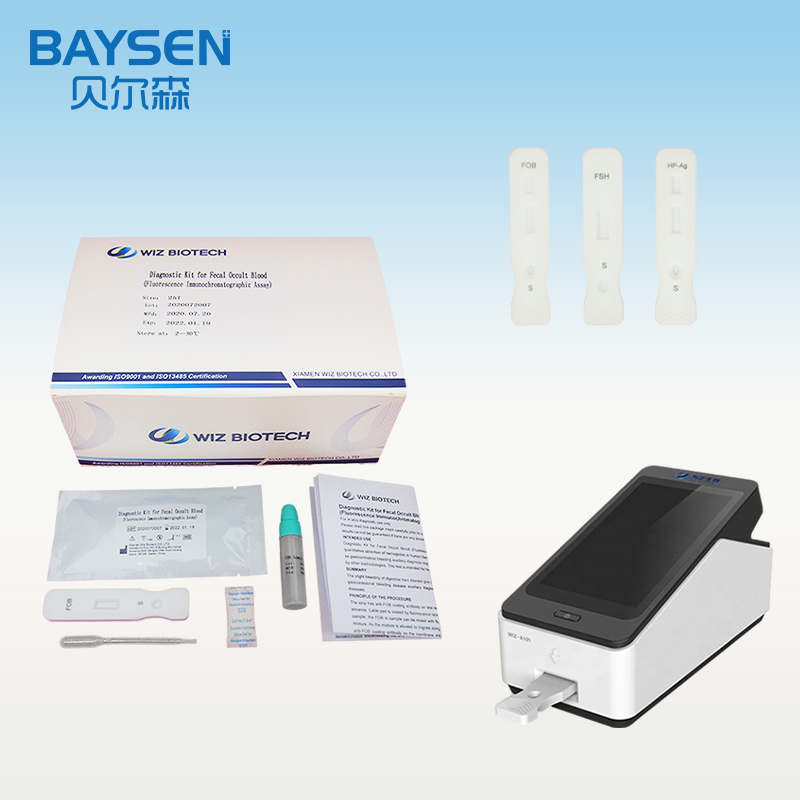
Manufactur standard Fob Psa Cea Afp Test Kit - Diagnostic Kit(Colloidal Gold)for Human Chorionic Gonadotrophin – Baysen
Manufactur standard Fob Psa Cea Afp Test Kit - Diagnostic Kit(Colloidal Gold)for Human Chorionic Gonadotrophin – Baysen Detail:
Diagnostic Kit(Colloidal Gold)for Human Chorionic Gonadotrophin
For in vitro diagnostic use only
Please read this package insert carefully prior to use and strictly follow the instructions. Reliability of assay results cannot be guaranteed if there are any deviations from the instructions in this package insert.
INTENDED USE
Diagnostic Kit(Colloidal Gold)for Human Chorionic Gonadotrophin is a colloidal gold immunochromatographic assay for the qualitative detection of human chorionic gonadotropin (HCG) levels in human serum and urine, it is used for early pregnancy diagnosis This test is a screening reagent. All positive sample must be confirmed by other methodologies. This test is intended for healthcare professional use only.
PACKAGE SIZE
1 kit /box, 10 kits /box, 25 kits,/box, 50 kits /box.
SUMMARY
HCG is a glycoprotein hormone secreted by the developing placenta after egg fertilization. HCG levels can be rapidly elevated in serum or urine as early as 1 to 2.5 weeks during pregnancy, and reach a peak in 8 week, than fall to medium level in 4 month, and maintained the level until the end of the pregnancy[1]. The Kit is a simple, visual qualitative test that detects HCG antigen in human serum or urine. The Diagnostic Kit is based on immunochromatography and can give a result within 15 minutes.
ASSAY PROCEDURE
1.Take out the test card from the foil bag, put it on the level table and mark it.
2.Discard the first two drops sample, add 3 drops (about 100μL) no bubble sample verticaly and slowly into sample well of the card with provided dispette, start timing.
3.The result should be read within 10-15 minutes, and it is invalid after 15 minutes.
Product detail pictures:

Related Product Guide:
Silencing MicroRNA-155 Attenuates Cardiac Injury and Dysfunction in Viral Myocarditis via Promotion of M2 Phenotype Polarization of Macrophages | Calprotectin Elisa Kit
Why Dutch hospitals are so good at beating superbugs | Diagnostic Kit For Isoenzyme Mb Of C Reatine Kinase
With a complete scientific quality management system, good quality and good faith, we win good reputation and occupied this field for Manufactur standard Fob Psa Cea Afp Test Kit - Diagnostic Kit(Colloidal Gold)for Human Chorionic Gonadotrophin – Baysen , The product will supply to all over the world, such as: Canada, Italy, Comoros, Our professional engineering group will always be ready to serve you for consultation and feedback. We are able to also offer you with absolutely free samples to meet your requirements. Finest efforts will likely be produced to give you the ideal service and goods. For anyone who is thinking about our company and merchandise, please contact us by sending us emails or contact us quickly. As a way to know our merchandise and firm. lot more, you can come to our factory to find out it. We'll always welcome guests from all over the world to our business to build company relations with us. Please feel free to get in touch with us for business and we believe we are going to share the top trading practical experience with all our merchants.
The after-sale warranty service is timely and thoughtful, encounter problems can be resolved very quickly, we feel reliable and secure.