Minimum pretium (MOQ) humilis pro Sinensi apparatu probationis signorum cardiacorum diagnosticorum unius gradus
"Qualitas initio, honestas ut fundamentum, societas sincera et lucrum mutuum" est nostra sententia, ut iterum atque iterum creamus et excellentiam pro MOQ humili pro Sinensi Uno Gradu Diagnostico Cardiaco Marker Test Kit persequamur. Nostra producta et solutiones magnam popularitatem apud emptores nostros fruuntur. Clientes, consociationes societatum et amicos ex omnibus partibus mundi invitamus ut nobiscum contactum faciant et cooperationem ad commodum mutuum quaerant.
"Qualitas initio, honestas ut fundamentum, societas sincera et lucrum mutuum" est nostra idea, ut iterum atque iterum creemus et excellentiam persequamur.Examen Fabricatoris Cardiaci Sinensis, Examen Troponini IPlus decem annos experientiae in exportatione habemus, et merces nostrae in plus quam triginta terras toto orbe terrarum exportatae sunt. Semper principium servitii, clientis, qualitatis, et qualitatis productorum severi sumus. Visitationem tuam libenter accipimus!
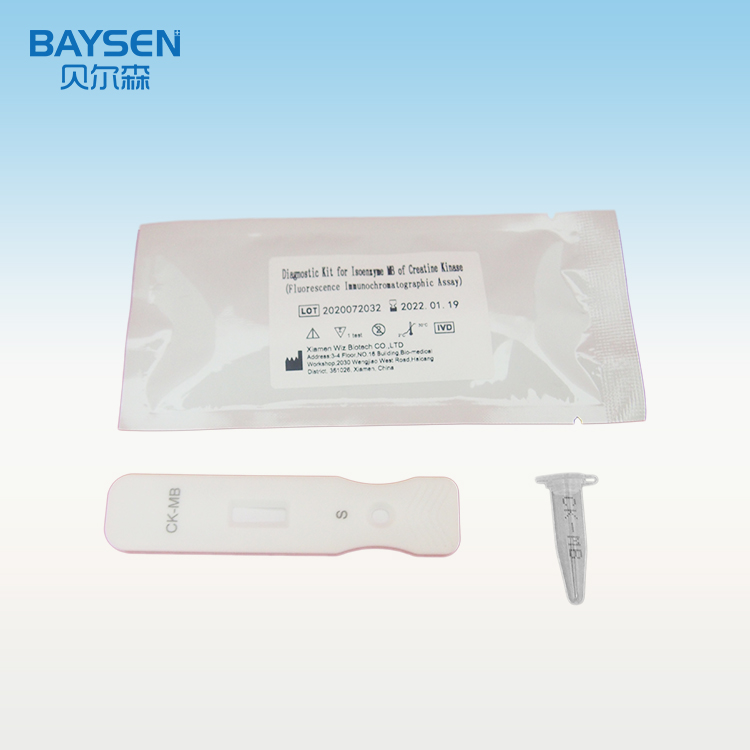
Libellus FOB



Principium et Ratio Probationis FOB
Principium:
Taenia anticorpus anti-FOB in regione probationis habet, quod antea chromatographiae membranae affixum est. Tegumentum inscriptio anticorpore anti-FOB fluorescentia notato antea obducitur. Cum exemplum positivum examinatur, FOB in exemplo cum anticorpore anti-FOB fluorescentia notato misceri potest, et mixturam immunem formare. Dum mixtura per taeniam migrare permittitur, complexus FOB coniugatus ab anticorpore anti-FOB in membrana capitur et complexum format. Intensitas fluorescentiae positive cum contento FOB correlata est. FOB in exemplo per analysatorem immunologicum fluorescentiae detegi potest.
Modus Probationis:
1. Omnia reagentia et exempla ad temperaturam cubiculi seponantur.
2. Aperi Analysorem Immunitatis Portatilem (WIZ-A101), inscribe tesseram inscriptionis secundum modum operationis instrumenti, et intra interfaciem detectionis.
3. Codicem identificationis inspice ut rem probatam confirmes.
4. Chartam probationis e sacculo aluminio extrahe.
5. Chartam probationis in fissuram chartae inser, codicem QR lege, et rem probatam determina.
6. Operculum e tubo speciminis remove et primas duas guttas speciminis diluti abice. Tres guttas (circiter 100µL) speciminis diluti sine bullis verticaliter et lente in puteum speciminis chartae cum dispense incluso adde.
7. Preme bullam "probationis ordinariae"; post XV minuta, instrumentum chartam probationis sponte deteget, eventus ex monitorio instrumenti legere, et eventus probationis notare/imprimere potest.
8. Instructiones Analysatoris Immunitatis Portatilis (WIZ-A101) vide.

Fortasse tibi placebit
Examen Celere Antigeni SARS-CoV-2 (Aurum Colloidale)
WIZ-A101 Analysator Immunitatis Portatilis
Instrumentum Diagnosticum Thyroxini Totalis (Examen Immunochromatographicum Fluorescens)
De Nobis

Xiamen Baysen Medical Tech Limited est societas biologica summae artis quae se in campo reagentium diagnosticorum celerium dedicat et investigationem et progressionem, productionem et venditionem in unum integrum integrat. Multi sunt in societate investigatores provecti et rectores venditionum, omnes qui amplam experientiam laboris in Sinis et societatibus biopharmaceuticis internationalibus habent.
Exhibitio certificati