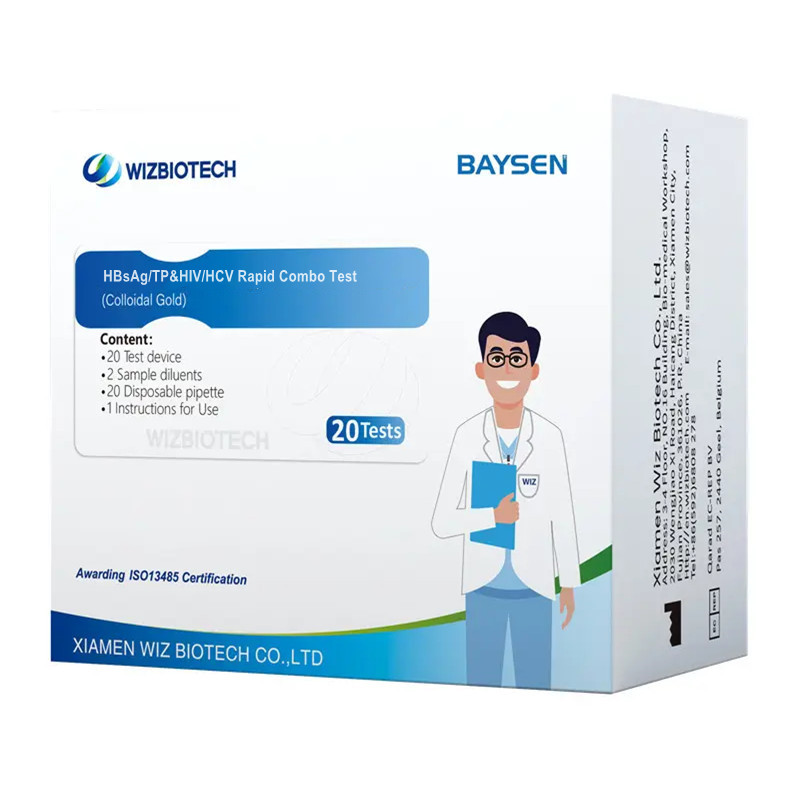
Examen Compositum Rapidum Infectiosum HIV HCV HBSAG ET Syphilismi
INFORMATIONES PRODUCTIONIS
| Numerus Modeli | HBsAg/TP et HIV/HCV | Sarcinatio | XX probationes/sarcina, XXX sarcinae/CTN |
| Nomen | Examen Compositum Celere HBsAg/TP et HIV/HCV | Classificatio instrumentorum | Classis III |
| Proprietates | Alta sensibilitas, facilis operatio | Certificatum | CE/ ISO13485 |
| Accuratio | > 97% | Tempus conservationis | Biennium |
| Methodologia | Aurum Colloidale | Officium OEM/ODM | Disponibile |
Superioritas
Tempus probationis: XV-XX minuta
Conservatio: 2-30℃/36-86℉
Methodologia: Aurum Colloidale
Proprietas:
• Alta sensibilitas
• Lectio eventus intra 15-20 minuta
• Facilis operatio
• Alta Praecisio
USUS INTENTUS
Hoc instrumentum aptum est ad determinationem qualitativam in vitro virus hepatitidis B, spirochetae syphilis, virus immunodeficientiae humanae, et virus hepatitidis C in sero/plasma humano.Exempla sanguinis totius/ma ad diagnosim auxiliarem infectionum virus hepatitidis B, spirochetae syphilis, virus immunodeficientiae humanae, et virus hepatitidis C. Resultata obtenta debentUna cum aliis notitiis clinicis analysari potest. Ad usum medicorum tantum peritorum destinatur.
Modus probationis
| 1 | Instructiones usus lege et operationem requisitam stricte observa, ne accuratio eventuum probationis afficiatur. |
| 2 | Ante experimentum, apparatus et exemplum e condicionibus repositionis extrahuntur et ad temperaturam ambientem aequantur et notantur. |
| 3 | Involucro sacculi aluminii scisso, instrumentum probationis extrahe et nota, deinde horizontaliter in mensa probationis pone. |
| 4 | Exempla seri/plasmatis guttatoribus disposable aspira et duas guttas in singulis puteis S1 et S2 adde; tres guttas in singulis puteis S1 et S2 pro exemplis sanguinis integri adde antequam unam vel duas guttas solutionis ablutionis in singulos puteos S1 et S2 addas et temporis mensuratio incipitur. |
| 5 | Resultata probationis intra XV~XX minuta interpretanda sunt; si plus quam XX minuta interpretata sunt, resultata irrita sunt. |
| 6 | Interpretatio visualis in interpretatione eventuum adhiberi potest. |
Nota: singula exempla pipetta munda et abicienda perfundenda sunt ne contaminatio mutua fiat.
EFFECTUS CLINICUS
| Resultata WIZ deHBsag
| Resultatum probationis reagentis referentialis | Ratio coincidentiae positivae: 99.06% (IC 95% 96.64% ~ 99.74%) Ratio coincidentiae negativae: 98.69% (IC 95%: 96.68% ~ 99.49%) Summa coincidentiae ratio: 98.84% (IC 95%: 97.50% ~ 99.47%) | ||
| Positivum | Negativum | Summa | ||
| Positivum | 211 | quattuor | 215 | |
| Negativum | Duo | 301 | 303 | |
| Summa | 213 | CCCV | 518 | |
| Resultata WIZ deTP
| Resultatum probationis reagentis referentialis | Ratio coincidentiae positivae: 96.18% (IC 95% 91.38% ~ 98.36%) Ratio coincidentiae negativae: 97.67% (IC 95%: 95.64% ~ 98.77%) Summa coincidentiae ratio: 97.30% (IC 95%: 95.51% ~ 98.38%) | ||
| Positivum | Negativum | Summa | ||
| Positivum | 126 | IX | 135 | |
| Negativum | quinque | 378 | 383 | |
| Summa | 131 | 387 | 518 | |
| Resultata WIZ deHCV
| Resultatum probationis reagentis referentialis | Ratio coincidentiae positivae: 93.44% (IC 95% 84.32% ~ 97.42%) Ratio coincidentiae negativae: 99.56% (IC 95%: 98.42% ~ 99.88%) Summa coincidentiae ratio: 98.84% (IC 95% 97.50% ~ 99.47%) | ||
| Positivum | Negativum | Summa | ||
| Positivum | 57 | Duo | 59 | |
| Negativum | quattuor | 455 | 459 | |
| Summa | LXI | 457 | 518 | |
| Resultata WIZ deHIV
| Resultatum probationis reagentis referentialis | Ratio coincidentiae positivae: 96.81% (IC 95% 91.03% ~ 98.91%) Ratio coincidentiae negativae: 99.76% (IC 95%: 98.68% ~ 99.96%) Summa coincidentiae ratio: 99.23% (IC 95%: 98.03% ~ 99.70%) | ||
| Positivum | Negativum | Summa | ||
| Positivum | 91 | 1 | 92 | |
| Negativum | Tres | 423 | 446 | |
| Summa | 94 | 424 | 518 | |