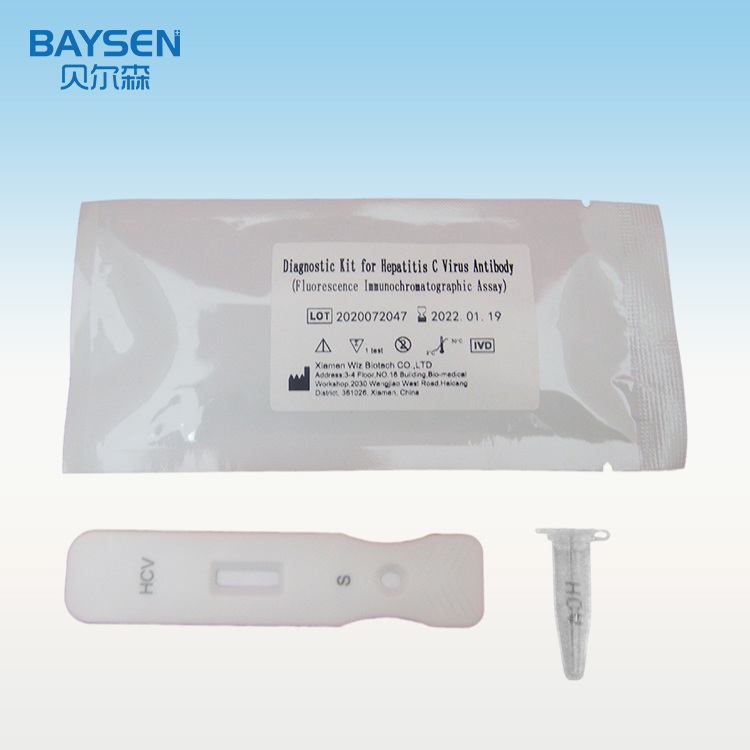
Instrumentum ad celerem probationem HCV uno gradu contra virus hepatitidis C
Parametri Productorum

Principium et Ratio Probationis FOB
PRINCIPIUM
Membrana instrumenti probationis antigeno HCV in regione probationis et anticorpore caprino anti-leporino IgG in regione moderatrice obducta est. Membrana inscriptionum antigeno HCV fluorescentia notato et IgG leporino antea obducta est. Cum exemplum positivum probatur, anticorpora HCV in exemplo cum antigeno HCV fluorescentia notato miscentur, mixturam immunem formant. Sub actione immunochromatographiae, complexus in directionem chartae absorbentis fluit; cum complexus regionem probationis transit, cum antigeno tegente antigeno HCV miscetur, novum complexum formans. Gradus anticorporis HCV positive cum signo fluorescentiae correlatur, et concentratio anticorporis HCV in exemplo per immunoassay fluorescentiae detegi potest.
Modus Probationis:
Quaeso, antequam experimentum facias, manuale operationis instrumenti et insertum involucri perlege.
1. Omnia reagentia et exempla ad temperaturam cubiculi seponantur.
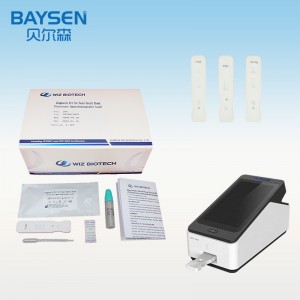
2. Aperi Analysorem Immunitatis Portatilem (WIZ-A101), inscribe tesseram inscriptionis secundum modum operationis instrumenti, et intra interfaciem detectionis.
3. Codicem identificationis lege ut rem probatam confirmes.
4. Chartam probationis e sacculo aluminio extrahe.
5. Chartam probationis in fissuram chartae inser, codicem QR lege, et rem probatam determina.
6. Adde 10μL seri vel plasmatis speciminis in diluentem speciminis, et bene misce.
7. 80μL solutionis speciminis in puteum speciminis chartae adde.
8. Preme bullam "probationis ordinariae"; post XV minuta, instrumentum chartam probationis sponte deteget, eventus ex monitorio instrumenti legere, et eventus probationis notare/imprimere potest.
9. Instructiones Analysatoris Immunitatis Portatilis (WIZ-A101) vide.

De Nobis

Xiamen Baysen Medical Tech Limited est societas biologica summae artis quae se in campo reagentium diagnosticorum celerium dedicat et investigationem et progressionem, productionem et venditionem in unum integrum integrat. Multi sunt in societate investigatores provecti et rectores venditionum, omnes qui amplam experientiam laboris in Sinis et societatibus biopharmaceuticis internationalibus habent.
Exhibitio certificati