Bonae Qualitatis Sinarum HCV Celeris Probationis Taenia/Cassetta Societatis Norma
Cum technologia nostra praestantissima una cum spiritu innovationis, cooperationis mutuae, beneficiorum et progressionis, futurum prosperum una cum vobis aestimatis societatibus pro Bona Qualitate Sinarum HCV Rapid Test Strip/Cassette Enterprise Standard aedificaturi sumus. Cum societas iuvenis et crescens simus, fortasse non optimi sumus, sed pro viribus conati sumus ut socii vestri optimi simus.
Cum technologia nostra praestantissima simul atque spiritu innovationis, cooperationis mutuae, beneficiorum et progressionis, futurum prosperum una cum vobis aestimatis aedificaturi sumus.Anti-HCV-Ns, Virus Hepatitis C SinensisSemper in principio administrationis "Qualitas prima, Ars fundamentum, Honestas et Innovatio" insistimus. Potuimus nova producta et solutiones continuo ad altiorem gradum evolvere, ut variis necessitatibus clientium satisfaciamus.
Ad usum diagnosticum in vitro tantum
Quaeso, ante usum, diligenter lege et instructiones stricte sequere. Fidelitas eventuum probationis garantiri non potest si quaelibet deviationes ab instructionibus in hac inscriptione factae sint.
USUS INTENTUS
Instrumentum Diagnosticum pro Anticorpore Virus Hepatitis C (Examen Immunochromatographicum Fluorescens) est examen immunochromatographicum fluorescentem ad detectionem quantitativam anticorporis HCV in sero vel plasma humano, quod est magni momenti valor diagnosticus auxiliaris pro infectione hepatitidis C. Omnia exempla positiva aliis methodologiis confirmanda sunt. Hoc examen solum ad usum professionalum curationis valetudinis destinatur.
1. Omnia reagentia et exempla ad temperaturam cubiculi seponantur.
2. Aperi Analysorem Immunitatis Portatilem (WIZ-A101), inscribe tesseram inscriptionis secundum modum operationis instrumenti, et intra interfaciem detectionis.
3. Codicem identificationis inspice ut rem probatam confirmes.
4. Chartam probationis e sacculo aluminio extrahe.
5. Chartam probationis in fissuram chartae inser, codicem QR lege, et rem probatam determina.
6. Adde 20μL seri vel plasmatis speciminis diluenti, et bene misce.
7. Adde 80μL solutionis speciminis in puteum speciminis chartae.
8. Preme bullam "probationis ordinariae"; post XV minuta, instrumentum chartam probationis sponte deteget, eventus ex monitorio instrumenti legere, et eventus probationis notare/imprimere potest.
9. Vide instructiones Analysatoris Immunitatis Portatilis (WIZ-A101).
SUMMARIUM
Virus hepatitidis C (HCV) est virus involucri, RNA positivo sensu singulari catenato (9.5 kb), ad familiam Flaviviridae pertinens. Sex genotypi maiores et series subtyporum HCV identificati sunt. Anno 1989 isolato, HCV nunc agnoscitur ut causa principalis hepatitidis non-A et non-B transfusione associatae. Morbus forma acuta et chronica insignitur. Plus quam 50% individuorum infectorum hepatitidem chronicam gravem, mortiferam, cum cirrhosi hepatis et carcinomatibus hepatocellularibus evolvunt. Ex quo anno 1990 examinatio anti-HCV in donationibus sanguinis introducta est, incidentia huius infectionis in recipientibus transfusionis significanter redacta est. Studia clinica ostendunt numerum significantem individuorum HCV infectorum anticorpora contra proteinum non-structurale NS5 virus evolvere. Ad hoc, probationes antigena ex regione NS5 genomatis viralis includunt, praeter NS3 (c200), NS4 (c200) et Core (c22).
PRINCIPIUM PROCEDURAE
Membrana instrumenti probationis antigeno HCV in regione probationis et anticorpore caprino anti-leporino IgG in regione moderatrice obducta est. Membrana inscriptionum antigeno HCV fluorescentia notato et IgG leporino antea obducta est. Cum exemplum positivum probatur, anticorpora HCV in exemplo cum antigeno HCV fluorescentia notato miscentur, mixturam immunem formant. Sub actione immunochromatographiae, complexus in directionem chartae absorbentis fluit; cum complexus regionem probationis transit, cum antigeno tegente antigeno HCV miscetur, novum complexum formans. Gradus anticorporis HCV positive cum signo fluorescentiae correlatur, et concentratio anticorporis HCV in exemplo per immunoassay fluorescentiae detegi potest.
REAGENTA ET MATERIAE SUPPEDITAE
Partes fasciculi 25T:
Charta probationis singula in sacculo metallico cum exsiccatore inclusa.
Diluentes speciminis
Insertio fasciculi
MATERIAE REQUISITAE SED NON PRAEBITAE
Vas collectionis speciminis, temporator
COLLECTIO ET CONSERVATIO EXEMPLORUM
1. Exempla examinata possunt esse serum, plasma heparini anticoagulans, vel plasma EDTA anticoagulans.
2. Secundum rationes consuetas, exemplum collige. Serum vel plasmatis exemplum in armario frigorifico ad 2-8°C per 7 dies servari potest, cryopreservari autem infra -15°C per 6 menses.
3. Omnia exempla cyclos congelationis et liquefactionis vitant.
PROCEDURA EXAMINATIONIS
Quaeso, antequam experimentum facias, manuale operationis instrumenti et insertum involucri perlege.
Hoc experimentum solum ad referentiam clinicam est, non solum ut fundamentum diagnosis clinicae et curationis praebere debet; curatio clinica aegroti comprehensiva consideranda est, una cum symptomatibus, historia medica, aliis examinationibus laboratorium, responsione ad curationem, epidemiologia, aliisque notitiis.
Hoc reagens solum ad probationes seri et plasmatis adhibetur. Fortasse non accurate resultat cum ad alia exempla, ut salivam et urinam, et cetera, adhibetur.
Proprietates Perfunctionis
| Linearitas | 0.005-5 | deviatio relativa: -15% ad +15%. |
| Coefficiens correlationis linearis: (r) ≥ 0.9900 | ||
| Accuratio | Ratio recuperationis intra 85% – 115% erit. | |
| Repetibilitas | CV≤15% | |
REFERENTIAE
1. Hepatitis post transfusionem. In: Moore SB, ed. Morbi Virales Transfusione Transmissi. Alington, VA. Am. Assoc. Bankae Sanguinis, pp. 53-38.
2. Hansen JH, et al. HAMA Interferentia cum Immunoassayis Murinis Fundatis in Anticorporibus Monoclonalibus [J]. J of Clin Immunoassay, 1993, 16:294-299.
3. Levinson SS. Natura Anticorporum Heterophilicorum et Munus in Interferentia Immunoassay [J]. J of Clin Immunoassay, 1992, 15:108-114.
4. Alter HJ., Purcell RH, Holland PV, et al. (1978) Agens transmissibile in hepatitide non-A, non-B. Lancet I: 459-463.
5. Choo QL, Weiner AJ, Overby LR, Kuo G, Houghton M. (1990) Virus Hepatitidis C: causa principalis hepatitidis viralis non-A, non-B. Br Med Bull 46: 423-441.
6. Engvall E, Perlmann P. (1971) Examen immunosorbens enzymaticum (ELISA): examen qualitativum IgG. Immunochemistry 8:871-874.
VALORES EXPECTATI
HCV-Ab<0.02
Suadetur ut unumquodque laboratorium suum proprium ambitum normalem constituat, qui suam aegrotorum multitudinem repraesentat.
RESULTATA PROBATIONUM ET INTERPRETATIO
- Data supradicta ex probatione reagentis HCV-Ab proveniunt, et suadetur ut unumquodque laboratorium seriem valorum detectionis HCV-Ab aptam populationi in hac regione constituat. Resultata supradicta tantum ad referentiam sunt.
- Resultata huius methodi solum ad intervalla referentialia in hac methodo statuta applicantur, neque ulla comparatio directa cum aliis methodis datur.
- Aliae causae etiam errores in eventibus detectionis efficere possunt, inter quas causae technicae, errores operationales, et alii factores exemplorum.
CONSERVATIO ET STABILITAS
- Instrumentum a die fabricationis per menses duodeviginti valet. Instrumenta non usata inter 2-30°C serva. NE CONGELES. Ne ultra diem expirationis utaris.
- Sacculum sigillatum ne aperias donec paratus sis ad experimentum perficiendum, et experimentum semel tantum adhibendum suadetur ut sub ambitu requisito (temperatura 2-35℃, humiditas 40-90%) intra 60 minuta quam celerrime adhibeatur.
- Diluens speciminis statim post aperturam adhibetur.
MONITIONES ET CAUTIONES
Instrumentum obsignandum et contra humiditatem protegendum est.
Omnia specimina positiva aliis methodologiis validanda sunt.
.Omnia specimina ut polluens potentiale habenda sunt.
.Reagento exspirato NOLI uti.
.Reagentia inter apparatus cum diversis numeris sortis NE permutare debes.
.Chartas probationum et accessiones abiciendas NE iterum utaris.
.Mala operatio, nimium vel parvum exemplum ad deviationes eventuum ducere potest.
LIMITATIO
.Sicut in quolibet experimento anticorpora murina adhibente, possibilitas interferentiae ab anticorporibus humanis anti-muribus (HAMA) in specimine existit. Specimina ab aegris qui praeparationes anticorporum monoclonalium ad diagnosim vel therapiam acceperunt HAMA continere possunt. Talia specimina eventus falsos positivos vel falsos negativos causare possunt.
Clavis symbolorum adhibitorum:
 | Instrumentum Medicum Diagnosticum In Vitro |
 | Fabricator |
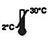 | Conserva apud 2-30℃ |
 | Dies Expirationis |
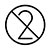 | Noli iterum uti |
 | CAUTIO |
 | Instructiones ad Usum Consule |