Instrumentum Diagnosticum Troponini I Cardiaci (examen immunochromatographicum fluorescentiae)
Instrumentum Diagnosticum Troponini Cardiaci I(examen immunochromatographicum fluorescentiae)
Ad usum diagnosticum in vitro tantum
Quaeso, ante usum, diligenter lege et instructiones stricte sequere. Fidelitas eventuum probationis garantiri non potest si quaelibet deviationes ab instructionibus in hac inscriptione factae sint.
USUS INTENTUS
Instrumentum diagnosticum pro Troponino Cardiaco I (examen immunochromatographicum fluorescentiae) est examen immunochromatographicum fluorescentiae ad detectionem quantitativam Troponini Cardiaci I (cTnI) in sero vel plasma humano, adhibetur ad diagnosin auxiliarem AMI (Infarctus Myocardii Acuti). Omnia exempla positiva aliis methodologiis confirmanda sunt. Hoc examen solum ad usum professionalum curandae valetudinis destinatur.
SUMMARIUM
Gradus cTnI aliquot horis post infarctionem myocardii aucti sunt, culmen attigerunt horis 12-16, et elevati manebant diebus 4-9 post infarctionem myocardii. Definitio globalis tertii infarctus myocardii anno 2012: Biomarker praeferendus - cTn (I vel T), magnam specificitatem textus myocardii et magnam sensibilitatem clinicam habet. Mutationes in concentratione cTn essentiales sunt ad diagnosin infarctus myocardii atrialis (IMA).
PRINCIPIUM PROCEDURAE
Membrana instrumenti probationis anticorpore anti-cTnI in regione probationis et anticorpore caprino anti-leporino IgG in regione moderatrice obducta est. Laminae inscriptionales anticorpore anti-cTnI fluorescentia notato et IgG leporino antea obducuntur. Cum exemplum positivum probatur, antigenum cTnI in exemplo cum anticorpore anti-cTnI fluorescentia notato miscetur, mixturam immunem formans. Sub actione immunochromatographiae, complexus in directionem chartae absorbentis fluit, et cum complexus regionem probationis transit, cum anticorpore anti-cTnI obducto miscetur, novum complexum formans. Gradus cTnI positive cum signo fluorescentiae correlatus est, et concentratio cTnI in exemplo per immunoassay fluorescentiae detegi potest.
REAGENTA ET MATERIAE SUPPEDITAE
Partes fasciculi 25T:
Charta probationis singula in sacculo metallico cum exsiccatore 25T inclusa
Diluentes speciminis 25T
Insertio fasciculi 1
MATERIAE REQUISITAE SED NON PRAEBITAE
Vas collectionis speciminis, temporator
COLLECTIO ET CONSERVATIO EXEMPLORUM
1. Exempla examinata possunt esse serum, plasma heparini anticoagulans, vel plasma EDTA anticoagulans.
2. Secundum rationes consuetas, exemplum collige. Serum vel plasmatis exemplum in armario frigorifico ad 2-8°C per 7 dies servari potest, cryopreservandum autem infra -15°C per 6 menses.
3. Omnia exempla cyclos congelationis et liquefactionis vitant.
PROCEDURA EXAMINATIONIS
Quaeso, antequam experimentum facias, manuale operationis instrumenti et insertum involucri perlege.
1. Omnia reagentia et exempla ad temperaturam cubiculi seponantur.
2. Aperi Analysorem Immunitatis Portatilem (WIZ-A101), inscribe tesseram inscriptionis secundum modum operationis instrumenti, et intra interfaciem detectionis.
3. Codicem identificationis inspice ut rem probatam confirmes.
4. Chartam probationis e sacculo aluminio extrahe.
5. Chartam probationis in fissuram chartae inser, codicem QR lege, et rem probatam determina.
6. Adde 40μL seri vel plasmatis speciminis diluenti, et bene misce.
7. Adde 80μL solutionis speciminis in puteum speciminis chartae.
8. Preme bullam "probationis ordinariae"; post XV minuta, instrumentum chartam probationis sponte deteget, eventus ex monitorio instrumenti legere, et eventus probationis notare/imprimere potest.
9. Vide instructiones Analysatoris Immunitatis Portatilis (WIZ-A101).
VALORES EXPECTATI
cTnI <0.3ng/mL
Suadetur ut unumquodque laboratorium suum proprium ambitum normalem constituat, qui suam aegrotorum multitudinem repraesentat.
RESULTATA PROBATIONUM ET INTERPRETATIO
Data supradicta ex probatione reagentis cTnI proveniunt, et suadetur ut unumquodque laboratorium seriem valorum detectionis cTnI aptam populationi in hac regione constituat. Resultata supradicta tantum ad referentiam sunt.
Resultata huius methodi solum ad intervalla referentialia in hac methodo constituta applicantur, neque ulla comparatio directa cum aliis methodis est.
Aliae causae etiam errores in detectionis eventibus efficere possunt, inter quas causae technicae, errores operationales, et alii factores exemplorum.
CONSERVATIO ET STABILITAS
1. Instrumentum a die fabricationis per 18 menses valet. Instrumenta non usata ad 2-30°C conserva. NE CONGELES. Ne ultra diem expirationis utaris.
2. Sacculum sigillatum ne aperias donec experimentum perficere paratus sis, et experimentum semel tantum adhibendum suadetur ut sub ambitu requisito (temperatura 2-35℃, humiditas 40-90%) intra 60 minuta quam celerrime adhibeatur.
3. Diluens speciminis statim post aperturam adhibetur.
MONITIONES ET CAUTIONES
Instrumentum obsignandum et contra humiditatem protegendum est.
Omnia specimina positiva aliis methodologiis validanda sunt.
Omnia specimina ut polluens potentiale habenda sunt.
Reagentum exspiratum NE utaris.
Reagentia inter apparatus cum diversis numeris sortis NE permutare debes.
Chartas probationum et accessiones abiciendas NE iterum adhibeas.
Mala operatio, nimium vel parvum exemplum ad deviationes eventuum ducere potest.
LIMITATIO
Sicut in quolibet experimento anticorpora murina adhibente, possibilitas interferentiae ab anticorporibus humanis anti-muribus (HAMA) in specimine existit. Specimina ab aegris qui praeparationes anticorporum monoclonalium ad diagnosim vel therapiam acceperunt HAMA continere possunt. Talia specimina eventus falsos positivos vel falsos negativos causare possunt.
Hoc experimentum solum ad referentiam clinicam est, non solum ut fundamentum diagnosis clinicae et curationis praebere debet; curatio clinica aegroti comprehensiva consideranda est, una cum symptomatibus, historia medica, aliis examinationibus laboratorium, responsione ad curationem, epidemiologia, aliisque notitiis.
Hoc reagens solum ad probationes seri et plasmatis adhibetur. Fortasse non accurate resultat cum ad alia exempla, ut salivam et urinam, et cetera, adhibetur.
Proprietates Perfunctionis
| Linearitas | 0.1ng/mL ad 40ng/mL | deviatio relativa: -15% ad +15%. |
| Coefficiens correlationis linearis: (r) ≥ 0.9900 | ||
| Accuratio | Ratio recuperationis intra 85% – 115% erit. | |
| Repetibilitas | CV≤15% | |
| Specificitas(Nulla substantiarum apud interferentem probatum in experimento impedivit.) | Interferens | Concentratio interferens |
| sTnI | 1000μg/L | |
| cTnT | 1000μg/L | |
| ABP | 1000μg/L | |
| CK-MB | 1000μg/L | |
| cTnC | 1000μg/L | |
| sTnT | 1000μg/L | |
| MYO | 1000μg/L | |
RREFERENTIAE
1. Hansen JH, et al. HAMA Interferentia cum Immunoassayis Murinis Fundatis in Anticorporibus Monoclonalibus [J]. J of Clin Immunoassay, 1993, 16:294-299.
2. Levinson SS. Natura Anticorporum Heterophilicorum et Munus in Interferentia Immunoassay [J]. J of Clin Immunoassay, 1992, 15:108-114.
Clavis symbolorum adhibitorum:
 | Instrumentum Medicum Diagnosticum In Vitro |
 | Fabricator |
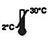 | Conserva apud 2-30℃ |
 | Dies Expirationis |
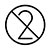 | Noli iterum uti |
 | CAUTIO |
 | Instructiones ad Usum Consule |
Xiamen Wiz Biotech Co., Ltd.
Inscriptio: Tabulatum 3-4, Aedificium 16, Officina Biomedica, Via Wengjiao Occidentalis 2030, Districtus Haicang, 361026, Xiamen, Sina.
Telephonum: +86-592-6808278
Fax: +86-592-6808279