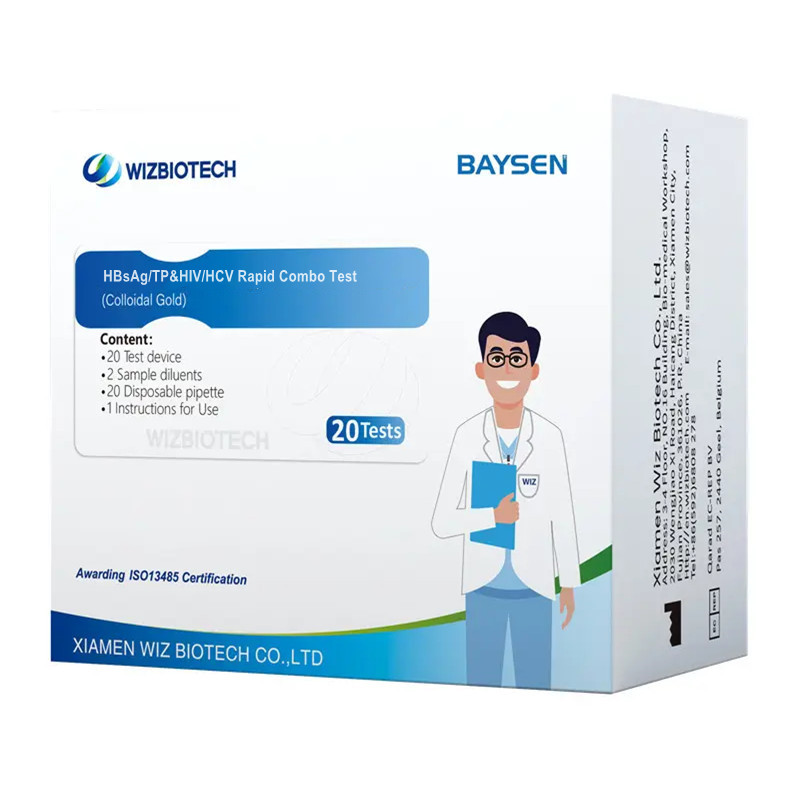
Infectious HIV HCV HBSAG AND Syphilish Rapid Combo Test
PRODUCTION INFORMATION
| Model Number | HBsAg/TP&HIV/HCV | Packing | 20 Tests/ kit, 30kits/CTN |
| Name | HBsAg/TP&HIV/HCV Rapid Combo Test |
Instrument classification | Class III |
| Features | High sensitivity, Easy opeation | Certificate | CE/ ISO13485 |
| Accuracy | > 97% | Shelf life | Two Years |
| Methodology | Colloidal Gold | OEM/ODM service | Avaliable |
Superiority
Testing time:15-20mins
Storage:2-30℃/36-86℉
Methodology:Colloidal Gold
Feature:
• High sensitive
• result reading in 15-20 minutes
• Easy operation
• High Accuracy
INTENDED USE
This kit is suitable for the in vitro qualitative determination of hepatitis B virus, syphilis spirochete, human immunodeficiency virus, and hepatitis C virus in human serum/plas- ma/whole blood samples for the auxiliary diagnosis of hepatitis B virus, syphilis spirochete, human immunodeficiency virus, and hepatitis C virus infections. The results obtained should be analyzed in conjunction with other clinical information. It is intended for use by medical professionals only.
Test procedure
| 1 | Read the instruction for use and in strict conformity with instruction for use required operation to avoid affecting the accuracy of the test results |
| 2 | Before the test, the kit and the sample are taken out from thestorage condition and balanced to room temperature and mark it. |
| 3 | Tearing the packaging of the aluminum foil pouch, take out the test device and mark it, then place it horizontally on the test table. |
| 4 | Aspirate serum/plasma samples with a disposable dropper and add 2 drops in each of wells s1 and s2; add 3 drops in each of wells s1 and s2 for whole blood samples before adding 1~2 drops of rinse solution to each of wells s1 and s2 and the Timing is started |
| 5 | Test results should be interpreted within 15~20 minutes, if more than 20 min interpreted results are invalid. |
| 6 | Visual interpretation can be used in result interpretation. |
Note: each sample shall be pipetted by clean disposable pipette to avoid cross contamination.
CLINICAL PERFORMANCE
| WIZ Results ofHBsag
|
Test result of Reference reagent | Positive coincidence rate:99.06% (95%C.I. 96.64%~99.74%) Negative coincidence rate:98.69% (95%C.I.96.68%~99.49%) Total coincidence rate:98.84% (95%C.I.97.50%~99.47% |
||
| Positive | Negative | Total | ||
| Positve | 211 | 4 | 215 | |
| Negative | 2 | 301 | 303 | |
| Total | 213 | 305 | 518 | |
| WIZ Results ofTP
|
Test result of Reference reagent | Positive coincidence rate:96.18% (95%C.I. 91.38%~98.36%) Negative coincidence rate:97.67% (95%C.I.95.64%~98.77%) Total coincidence rate:97.30% (95%C.I.95.51%~98.38%) |
||
| Positive | Negative | Total | ||
| Positve | 126 | 9 | 135 | |
| Negative | 5 | 378 | 383 | |
| Total | 131 | 387 | 518 | |
| WIZ Results ofHCV
|
Test result of Reference reagent | Positive coincidence rate:93.44% (95%C.I. 84.32%~97.42%) Negative coincidence rate:99.56% (95%C.I.98.42%~99.88%) Total coincidence rate:98.84% (95%C.I.97.50%~99.47%) |
||
| Positive | Negative | Total | ||
| Positve | 57 | 2 | 59 | |
| Negative | 4 | 455 | 459 | |
| Total | 61 | 457 | 518 | |
| WIZ Results ofHIV
|
Test result of Reference reagent | Positive coincidence rate:96.81% (95%C.I. 91.03%~98.91%) Negative coincidence rate:99.76% (95%C.I.98.68%~99.96%) Total coincidence rate:99.23% (95%C.I.98.03%~99.70%) |
||
| Positive | Negative | Total | ||
| Positve | 91 | 1 | 92 | |
| Negative | 3 | 423 | 446 | |
| Total | 94 | 424 | 518 | |