High Performance Rapid Tb Diagnostic Test Kit Fda Cleared Ce Mark - Diagnostic Kit (Colloidal Gold) for IgM/IgG Antibody to Dengue Virus – Baysen
High Performance Rapid Tb Diagnostic Test Kit Fda Cleared Ce Mark - Diagnostic Kit (Colloidal Gold) for IgM/IgG Antibody to Dengue Virus – Baysen Detail:
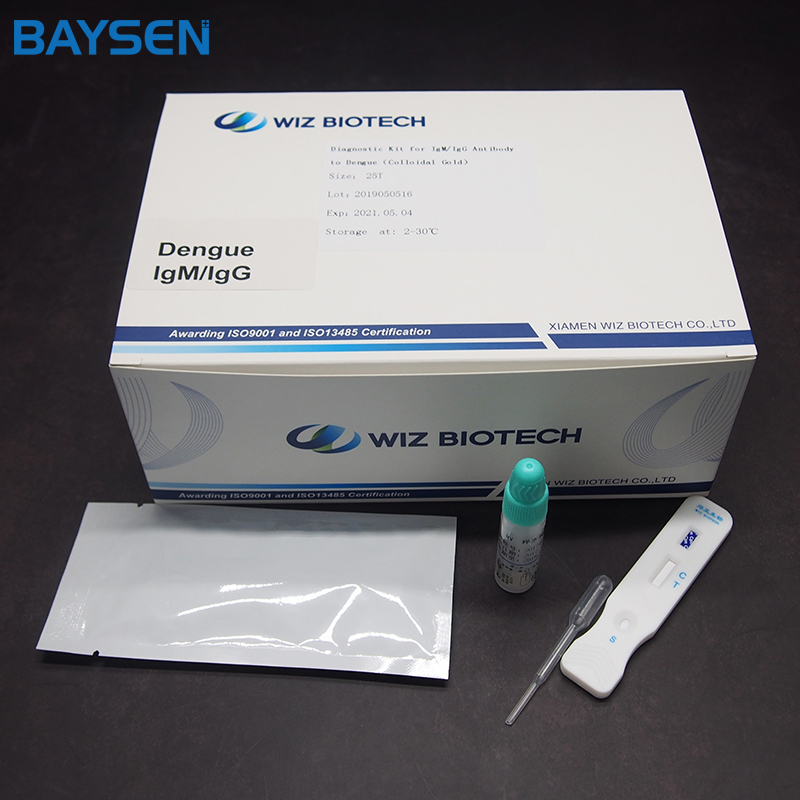
Diagnostic Kit (Colloidal Gold) for IgM/IgG Antibody to Dengue Virus
For in vitro diagnostic use only
Please read this package insert carefully prior to use and strictly follow the instructions. Reliability of assay results cannot be guaranteed if there are any deviations from the instructions in this package insert.
INTENDED USE
Diagnostic Kit(Colloidal Gold)for IgM/IgG Antibody to Dengue Virus is a colloidal gold immunochromatographic assay for the qualitative detection of IgM/IgG Antibody to Dengue Virus in human serum and plasma. This test is a screening reagent. All positive sample must be confirmed by other methodologies. This test is intended for healthcare professional use only.
PACKAGE SIZE
Twenty-five serving.
SUMMARY
Dengue Fever (Dengue Fever) is caused by Dengue virus infection. Dengue Fever is a group B vector virus and the yellow virus family flavivirus. Dengue virus antibody tests are often used enzyme-linked Immunoassay, Indirect Immunofluorescent Assay, Immunochromato – graphic Test. In recent years, with the development of the immune colloidal gold chromatography technology, using colloidal gold immune chromatography to detect serum (plasma) dengue virus antibody has become a reality. This method is characterized by simple operation, quick detection speed and saving manpower and material resources. It makes up for the inconveniences of ELISA and chemiluminescence method.
ASSAY PROCEDURE
1.Take out the test card from the foil bag, put it on the level table and mark it.
2.Use pipette to absorb sample 2ul, add it into sample well of the card;
3.Add 2 drops (80-100ul) sample diluent into sample well of the card;
4.The result should be read within 20 minutes.
TEST RESULTS AND INTERPRETATION[2]
Product detail pictures:


Related Product Guide:
Major Trial: No Mortality Benefit With ‘One-Off’ PSA Test 3090D553-9492-4563-8681-AD288FA52ACE Group 2 34A8E98B-62ED-4216-98D6-E986304F4C2E | Psa Test Cost
Payback time for prostate cancer tests | Calprotectin Elisa Kit
The incredibly rich projects administration experiences and a person to 1 service model make the substantial importance of organization communication and our easy understanding of your expectations for High Performance Rapid Tb Diagnostic Test Kit Fda Cleared Ce Mark - Diagnostic Kit (Colloidal Gold) for IgM/IgG Antibody to Dengue Virus – Baysen , The product will supply to all over the world, such as: Maldives, Roman, India, If any product meed your demand, remember to feel free to contact us. We're sure your any inquiry or requirement will get prompt attention, high-quality merchandise, preferential prices and cheap freight. Sincerely welcome friends all over the world to call or come to visit, to discuss cooperation for a better future!
The customer service reprersentative explained very detailed, service attitude is very good, reply is very timely and comprehensive, a happy communication! We hope to have a opportunity to cooperate.