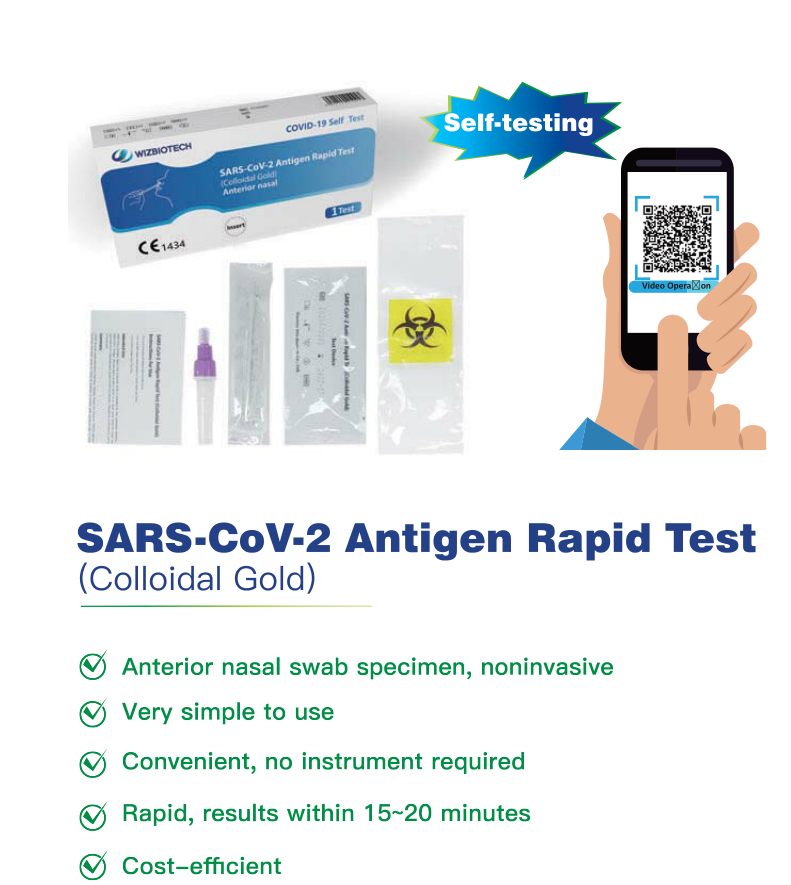
Mylasia ta amince da SARS-CoV-2 antigen saurin gwajin kayan gwajin kai
Mylasia ta amince da SARS-CoV-2 antigen saurin gwajin kayan gwajin kai
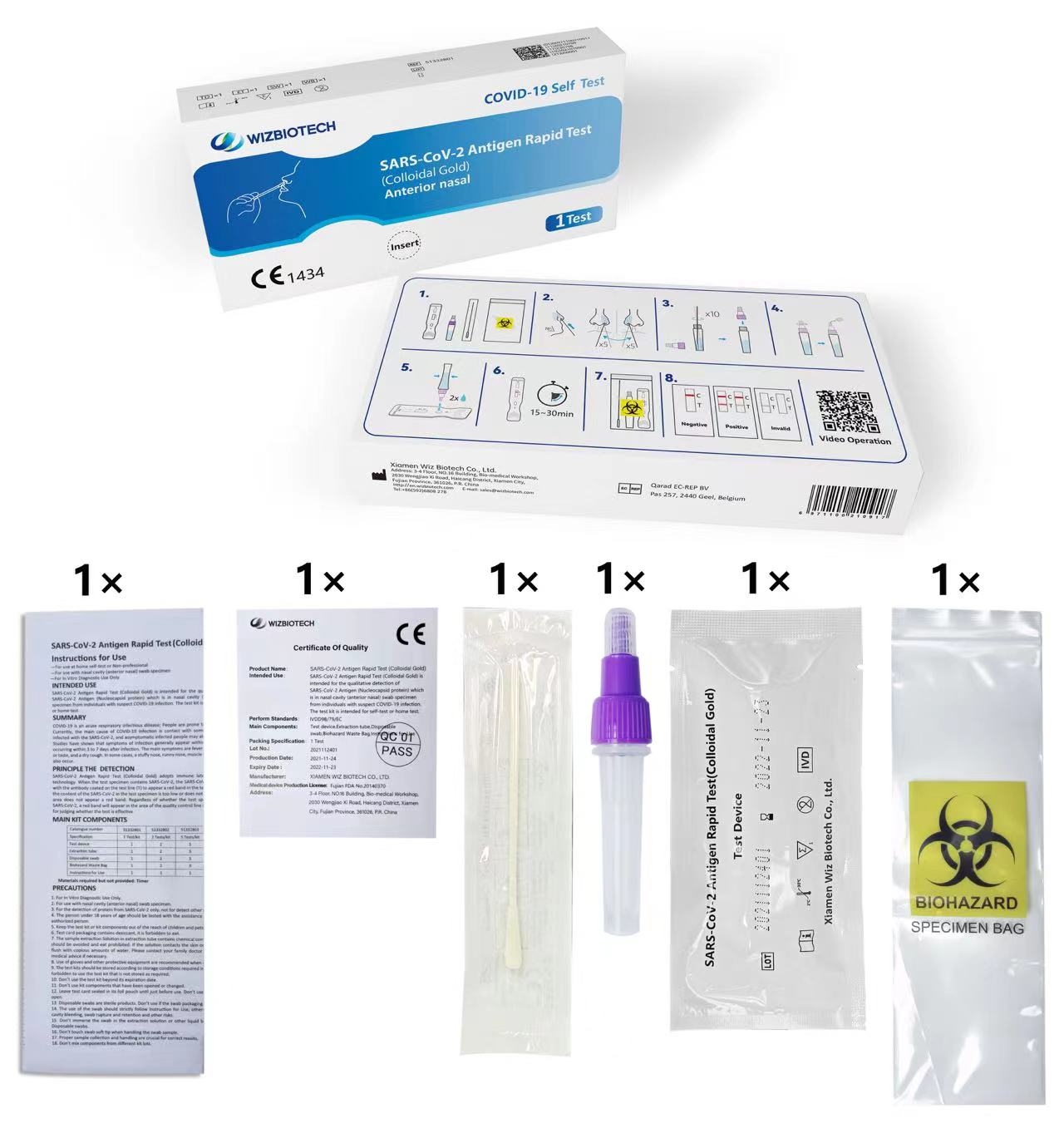
Umarnin don Amfani
- don amfani a gida
jarrabawar kai ko mara sana'a
-Don amfani da kogon hanci (nasal na gaba) samfurin swab
- Don Amfanin Bincike na In Vitro kawai
Adana
Ya kamata a adana kayan gwaji na 2°C ~ 30°C, bushewa kuma daga hasken rana kai tsaye (Kada a daskare kayan ko kayan aikinta).
Rayuwar shiryayye na kit ɗin shine watanni 12.
Ya kamata a yi amfani da katin gwajin a cikin mintuna 60 bayan buɗe jakar foil na aluminum.
Don kwanan ranar karewa kit, da fatan za a koma kan alamar samfur.
Hankali: 98.26% (95% CI 93.86% ~ 99.79%)
Musamman: 100.00% (95% CI 99.19% ~ 100.00%)
Kyakkyawan Hasashen Hasashen: 100% (95% CI 96.79% ~ 100.00%)
Ƙimar Hasashen Ƙira: 99.56% (95% CI 98.43% ~ 99.95%)
Yarjejeniyar Kashi Gabaɗaya: 99.65% (95% CI 98.74 ~ 99.96%)
Gwajin gaggawa na SARS-CoV-2 an yi niyya ne don gano ƙimar SARS-CoV-2 Antigen a cikin swab oropharyngea da samfuran swab na nasopharyngeal a cikin Vitro.