Kyawawan Ingancin China HCV Saurin Gwajin Gwajin Sauri/ Matsayin Kasuwancin Cassette
Tare da mu manyan fasahar a lokaci guda kamar yadda mu ruhu na bidi'a, juna hadin gwiwa, amfani da ci gaba, za mu gina wani m makoma tare da juna tare da kimar sha'anin ga Good Quality kasar Sin HCV Rapid Test Strip / Cassette Enterprise Standard, Kasancewa matasa karuwa kungiyar, za mu iya ba mafi kyau, amma mun kasance kokarin mu mafi kyau ga zama your sosai kyau abokin tarayya.
Tare da manyan fasahar mu a lokaci guda da ruhin mu na ƙididdigewa, haɗin gwiwar juna, fa'idodi da ci gaba, za mu gina makoma mai wadata tare da juna tare da manyan kasuwancin ku.Anti-HCV-Ns, China Hepatitis C Virus, Mu ko da yaushe nace a kan management tenet na "Quality ne na farko, Technology ne tushen, Gaskiya da Innovation" .Mun sami damar ci gaba da sababbin samfurori da kuma mafita ci gaba da girma matakin don gamsar daban-daban bukatun abokan ciniki.
Don bincikar in vitro amfani kawai
Da fatan za a karanta wannan fakitin a hankali kafin amfani kuma a bi umarnin sosai. Ba za a iya tabbatar da amincin sakamakon kima ba idan akwai wasu sabani daga umarnin a cikin wannan fakitin.
AMFANI DA NUFIN
Kit ɗin Diagnostic don Hepatitis C Virus Antibody (Fluorescence Immunochromatographic Assay) shine gwajin immunochromatographic mai haske don gano ƙididdigar adadin HCV a cikin jini ko plasma, wanda yake da mahimmancin ƙimar bincike don kamuwa da cutar hepatitis C. Duk samfuran tabbatacce dole ne a tabbatar da su ta wasu hanyoyin. Anyi nufin wannan gwajin don amfanin ƙwararrun kiwon lafiya kawai
1.Lay a gefe duk reagents da samfurori zuwa dakin zafin jiki.
2.Bude Portable Immune Analyzer (WIZ-A101), shigar da kalmar sirri shiga asusu bisa ga tsarin aiki na kayan aiki, da kuma shigar da ganowa dubawa.
3.Scan da lambar haƙori don tabbatar da gwajin abu.
4.Dauki katin gwaji daga jakar foil.
5.Saka katin gwaji a cikin ramin katin, duba lambar QR, kuma ƙayyade abin gwajin.
6.Add 20μL serum ko plasma samfurin zuwa samfurin diluent, da kuma Mix da kyau ..
7.Add 80μL samfurin bayani don samfurin rijiyar katin.
8. Danna maɓallin "gwajin misali", bayan minti 15, kayan aiki za su gano katin gwajin ta atomatik, zai iya karanta sakamakon daga allon nuni na kayan aiki, kuma rikodin / buga sakamakon gwajin.
9. Koma zuwa ga umarnin Portable Immune Analyzer (WIZ-A101).
TAKAITACCEN
Kwayar cutar Hepatitis C (HCV) wani ambulan ne, kwayar cuta mai ma'ana guda RNA (9.5kb) na dangin Flaviviridae. An gano manyan genotypes shida da jerin nau'ikan nau'ikan HCV. An keɓe shi a cikin 1989, HCV yanzu an gane shi a matsayin babban dalilin zubar da jini da ke hade da wadanda ba A, ba na B. Cutar da aka kwatanta da m da na kullum nau'i. Fiye da kashi 50 cikin 100 na mutanen da suka kamu da cutar suna tasowa mai tsanani, suna barazanar rayuwa mai cutar hanta tare da hanta cirrhosis da carcinomas na hepatocellular. Tun bayan gabatarwa a cikin 1990 na gwajin anti-HCV na gudummawar jini, an rage yawan kamuwa da wannan cuta a cikin masu karɓar ƙarin jini sosai. Nazarin asibiti ya nuna cewa yawancin mutanen da suka kamu da cutar ta HCV suna haɓaka ƙwayoyin rigakafi zuwa furotin na NS5 marasa tsari na ƙwayar cuta. Don wannan, gwaje-gwajen sun haɗa da antigens daga yankin NS5 na kwayar cutar kwayar cuta ban da NS3 (c200), NS4 (c200) da Core (c22).
KA'IDAR HANYA
An lulluɓe jikin na'urar gwajin tare da antigen HCV akan yankin gwajin da goat anti-zomo IgG antibody akan yankin sarrafawa. Label pad an lullube shi ta hanyar kyalli mai suna HCV antigen da zomo IgG a gaba. Lokacin gwada ingantaccen samfurin, maganin rigakafi na HCV a cikin samfurin yana haɗuwa da haske mai suna HCV antigen, kuma ya samar da cakuda rigakafi. A karkashin aikin da immunochromatography, da hadaddun ya kwarara a cikin shugabanci na absorbent takarda, a lokacin da hadaddun wuce gwajin yankin, shi a hade tare da HCV antigen shafi antigen, Forms sabon hadaddun.HCV antibody matakin da gaskiya da alaka da kyalli siginar, da taro na HCV antibody a cikin samfurin za a iya gano ta fluorescence immunoassay assay.
REAgents DA KAYAN DA AKA BAYAR
Abubuwan kunshin 25T:
.Katin gwajin ɗaiɗaiku foil ɗin da aka shayar da shi tare da na'urar bushewa
.Sample diluents
. Kunshin saka
KAYAN DA AKE BUKATA AMMA BA'A SAMU BA
Samfurin tarin akwati, mai ƙidayar lokaci
MISALI TATTAUNAWA DA AJIYA
1. Samfuran da aka gwada na iya zama jini, heparin anticoagulant plasma ko EDTA anticoagulant plasma.
2.According ga daidaitattun dabarun tattara samfurin. Za'a iya ajiye samfurin jini ko plasma a cikin firiji a 2-8 ℃ na tsawon kwanaki 7 kuma a adana cryopreservation ƙasa -15 ° C na watanni 6.
3.All samfurin guje wa daskare-narke hawan keke.
HANYAR ASSAY
Da fatan za a karanta jagorar aiki na kayan aiki da saka fakiti kafin gwaji.
.Wannan sakamakon gwajin kawai don tunani na asibiti, bai kamata ya zama tushen kawai don ganewar asibiti da magani ba, masu kula da marasa lafiya ya kamata a yi la'akari da su sosai tare da alamunta, tarihin likita, sauran gwaje-gwaje na dakin gwaje-gwaje, amsawar magani, cututtukan cututtuka da sauran bayanai.
.Wannan reagent ana amfani dashi kawai don gwajin jini da jini. Maiyuwa baya samun ingantaccen sakamako idan aka yi amfani da shi don wasu samfura kamar yau da fitsari da sauransu.
HALAYEN YI
| Linearity | 0.005-5 | bambancin dangi: -15% zuwa +15%. |
| Matsakaicin daidaitawar layi:(r)≥0.9900 | ||
| Daidaito | Adadin dawowa zai kasance cikin 85% - 115%. | |
| Maimaituwa | CV≤15% | |
NASARA
1.Bayan jini hanta. A cikin: Moore SB, ed. Cututtukan Cutar Cutar Kwayar cuta. Alington, VA. Am. Assoc. Bankin Jini, shafi na 53-38.
2.Hansen JH, et al.HAMA Tsangwama tare da Murine Monoclonal Antibody-Based Immunoassays[J].J na Clin Immunoassay,1993,16:294-299.
3.Levinson SS.Yanayin Heterophilic Antibodies da Matsayin Tsangwama na Immunoassay[J].J na Clin Immunoassay,1992,15:108-114.
4.Alter HJ., Purcell RH, Holland PV, et al. (1978) wakili mai iya canzawa a cikin wadanda ba A, wadanda ba B. Lancet I: 459-463.
5.Choo QL, Weiner AJ, Overby LR, Kuo G, Houghton M. (1990) Cutar Hepatitis C: babban abin da ke haifar da kwayar cutar hanta wanda ba A, ba B. Br Med Bull 46: 423-441.
6.Engvall E, Perlmann P. (1971) Enzyme nasaba immunosorbent assay (ELISA): qualitative assay na IgG. Immunochemistry 8:871-874.
DABI'UN DA AKE SARANSU
HCV-Ab <0.02
Ana ba da shawarar cewa kowane dakin gwaje-gwaje ya kafa nasa kewayon al'ada wanda ke wakiltar yawan majinyacinsa.
SAKAMAKON JARRABAWA DA FASSARA
- Bayanan da ke sama sakamakon gwajin reagent HCV-Ab ne, kuma ana ba da shawarar cewa kowane dakin gwaje-gwaje ya kafa kewayon ƙimar gano HCV-Ab wanda ya dace da yawan jama'a a wannan yanki. Sakamakon da ke sama don tunani ne kawai.
- Sakamakon wannan hanyar yana aiki ne kawai ga jeri na tunani da aka kafa a wannan hanyar, kuma babu kwatankwacin kai tsaye da sauran hanyoyin.
- Sauran abubuwan kuma na iya haifar da kurakurai a cikin sakamakon ganowa, gami da dalilan fasaha, kurakurai na aiki da sauran abubuwan samfuri.
AJIYA DA KWANTA
- Kit ɗin shine rayuwar rayuwar watanni 18 daga ranar da aka yi. Ajiye kayan da ba a yi amfani da su ba a 2-30 ° C. KAR KA DANKE. Kar a yi amfani da bayan ranar karewa.
- Kada a buɗe jakar da aka hatimi har sai kun shirya don yin gwaji, kuma gwajin amfani guda ɗaya ana ba da shawarar a yi amfani da shi a ƙarƙashin yanayin da ake buƙata (zazzabi 2-35 ℃, zafi 40-90%) cikin mintuna 60 da sauri.
- Ana amfani da samfurin diluent nan da nan bayan an buɗe shi.
GARGADI DA TSIRA
.Kit ɗin yakamata a rufe shi kuma a kiyaye shi daga danshi.
.Duk samfurori masu inganci za a inganta su ta wasu hanyoyin.
.Duk samfuran za a kula da su azaman masu gurɓata yanayi.
.KADA KA yi amfani da reagent da ya ƙare.
.KADA KA musanya reagents tsakanin kits tare da kuri'a daban-daban No..
.KAR KA sake amfani da katunan gwaji da duk wani na'urorin da za'a iya zubarwa.
.Rashin aiki, wuce gona da iri ko ƙaramin samfurin na iya haifar da karkatattun sakamako.
LIMITATION
.Kamar yadda yake tare da kowane gwajin amfani da ƙwayoyin rigakafi na linzamin kwamfuta, akwai yuwuwar tsoma baki daga ƙwayoyin rigakafin linzamin kwamfuta na ɗan adam (HAMA) a cikin samfurin. Samfura daga marasa lafiya waɗanda suka karɓi shirye-shiryen rigakafin ƙwayoyin cuta na monoclonal don ganewar asali ko jiyya na iya ƙunsar HAMA. Irin waɗannan samfurori na iya haifar da sakamako mara kyau na ƙarya ko ƙarya.
Maɓalli ga alamomin da aka yi amfani da su:
 | In Vitro Diagnostic Medical Na'urar |
 | Mai ƙira |
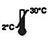 | Adana a 2-30 ℃ |
 | Ranar Karewa |
 | Kada a sake amfani |
 | HANKALI |
 | Tuntuɓi Umarnin Don Amfani |