Factory Supply Medical Pregnancy Test Kits - Diagnostic Kit(LATEX)for Rotavirus Group A – Baysen
Factory Supply Medical Pregnancy Test Kits - Diagnostic Kit(LATEX)for Rotavirus Group A – Baysen Detail:
Diagnostic Kit(LATEX)for Rotavirus Group A
For in vitro diagnostic use only
Please read this package insert carefully prior to use and strictly follow the instructions. Reliability of assay results cannot be guaranteed if there are any deviations from the instructions in this package insert.
INTENDED USE
Diagnostic Kit(LATEX)for Rotavirus Group A is suitable for qualitative detection of Rotavirus Group A antigen in human fecal samples. This test is intended for healthcare professional use only. Meanwhile, this test is used for the clinical diagnosis of infantile diarrhea in patients with Rotavirus Group A infection.
PACKAGE SIZE
1 kit /box, 10 kits /box, 25 kits,/box, 50 kits /box.
SUMMARY
Rotavirus is classified as a rotavirus genus of the exenteral virus, which has a spherical shape with a diameter of about 70nm. Rotavirus contains 11 segments of double-stranded RNA. The rotavirus can be seven groups (a-g) based on antigenic differences and gene characteristics. Human infections of group A, group B and C group rotavirus have been reported. Rotavirus Group A is the important cause of severe gastroenteritis in children worldwide[1-2].
ASSAY PROCEDURE
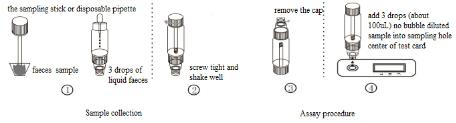
1.Take out the sampling stick, inserted into the faeces sample, then put the sampling stick back, screw tight and shake well, repeat the action 3 times. Or using the sampling stick picked about 50mg faeces sample, and put in a faeces sample tube containing sample dilution, and screw tightly.
2.Use disposable pipette sampling take the thinner faeces sample from the diarrhea patient, then add 3 drops (about 100uL) to the fecal sampling tube and shake well, put aside.
3.Take out the test card from the foil bag, put it on the level table and mark it.
4.Remove the cap from the sample tube and discard the first two drops diluted sample, add 3 drops (about 100uL) no bubble diluted sample verticaly and slowly into sample well of the card with provided dispette, start timing.
5.The result should be read within 10-15 minutes, and it is invalid after 15 minutes.
Product detail pictures:

Related Product Guide:
Heart Attack Diagnostics Market Poised To Reach $15.4 Billion By 2024: Grand View Research Inc. – Press Release | Calprotectin Elisa Kit
GPs should not screen patients over 70 for prostate cancer, researchers say | News Article | Diagnostic Kit For Isoenzyme Mb Of C Reatine Kinase
We emphasize advancement and introduce new products into the market each year for Factory Supply Medical Pregnancy Test Kits - Diagnostic Kit(LATEX)for Rotavirus Group A – Baysen , The product will supply to all over the world, such as: Thailand, Saudi Arabia, Paris, With top quality products, great after-sales service and warranty policy, we win trust from many overseas partner, many good feedbacks witnessed our factory's growth. With full confidence and strength, welcome customers to contact and visit us for future relationship.
It is really lucky to meet such a good supplier, this is our most satisfied cooperation, I think we will work again!