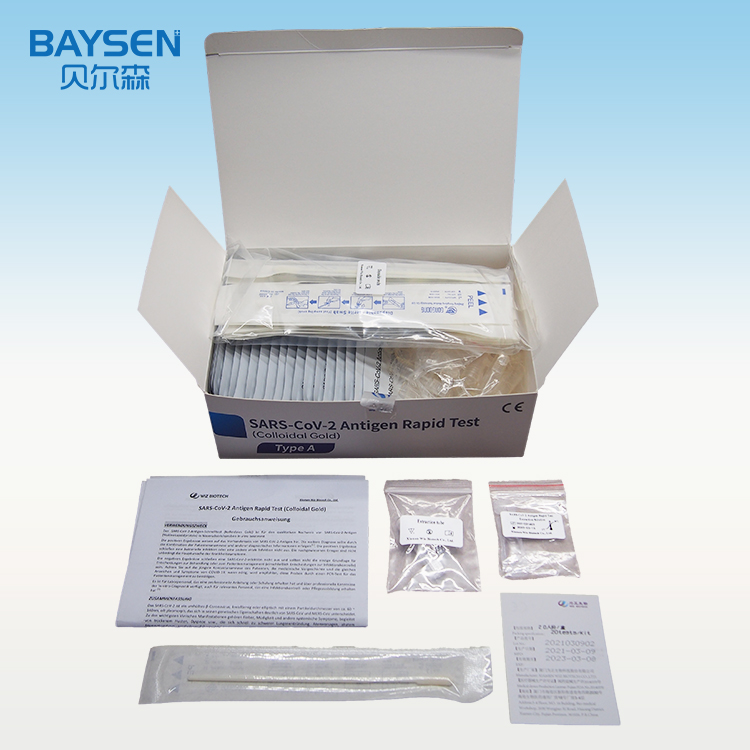
Factory source Tumorigenesis Test Kit - Diagnostic kit for C-reative protein (CRP) Quantitative Cassette – Baysen
Factory source Tumorigenesis Test Kit - Diagnostic kit for C-reative protein (CRP) Quantitative Cassette – Baysen Detail:
Diagnostic Kit for hypersensitive C-reactive protein
(fluorescence immunochromatographic assay)
For in vitro diagnostic use only
Please read this package insert carefully prior to use and strictly follow the instructions. Reliability of assay results cannot be guaranteed if there are any deviations from the instructions in this package insert.
INTENDED USE
Diagnostic Kit for hypersensitive C-reactive protein (fluorescence immunochromatographic assay) is a fluorescence immunochromatographic assay for the quantitative detection of C-reactive protein (CRP) in human serum /plasma/ Whole blood. It is a non-specific indicator of inflammation. All positive sample must be confirmed by other methodologies. This test is intended for healthcare professional use only.
SUMMARY
C-reactive protein is an acute phase protein produced by lymphokine stimulation of liver and epithelial cells. It exists in human serum, cerebrospinal fluid, pleural and abdominal fluid, etc., and is a part of non-specific immune mechanism. 6-8h after the occurrence of bacterial infection,CRP began to increase, 24-48h reached the peak, and the peak value could reach hundreds of times of normal. After the elimination of the infection, CRP dropped sharply and returned to normal within one week. However, CRP does not increase significantly in the case of viral infection, which provides a basis for the identification of early infection types of diseases, and is a tool for identifying viral or bacterial infections.
PRINCIPLE OF THE PROCEDURE
The membrane of the test device is coated with anti CRP antibody on the test region and goat anti rabbit IgG antibody on the control region. Lable pad are coated by fluorescence labeled anti CRP antibody and rabbit IgG in advance. When testing positive sample, the CRP antigen in sample combine with fluorescence labeled anti CRP antibody, and form immune mixture. Under the action of the immunochromatography, the complex flow in the direction of absorbent paper, when complex passed the test region, it combined with anti CRP coating antibody, forms new complex. CRP level is positively correlated with fluorescence signal, and the concentration of CRP in sample can be detected by fluorescence immunoassay assay.
REAGENTS AND MATERIALS SUPPLIED
25T package components:
Test card individually foil pouched with a desiccant 25T
Sample diluents 25T
Package insert 1
MATERIALS REQUIRED BUT NOT PROVIDED
Sample collection container,timer
SAMPLE COLLECTION AND STORAGE
- The samples tested can be serum, heparin anticoagulant plasma or EDTA anticoagulant plasma.
- According to standard techniques collect sample. Serum or plasma sample can be kept refrigerated at 2-8℃ for 7days and cryopreservation below -15°C for 6 months. Whole blood sample can be kept refrigerated at 2-8℃ for 3 days
- All sample avoid freeze-thaw cycles.
Product detail pictures:

Related Product Guide:
MDxHealth (R): SelectMDx Cost-effective in Four European Countries Brussels Stock Exchange:MDXH | Psa Test Cost
Older Men Gain Little From PSA Test: Study â WebMD | P24 Test Strips
We emphasize development and introduce new products into the market every year for Factory source Tumorigenesis Test Kit - Diagnostic kit for C-reative protein (CRP) Quantitative Cassette – Baysen , The product will supply to all over the world, such as: Singapore, Lahore, Uruguay, Now, with the development of internet, and the trend of internationalization, we've got decided to extend business to overseas market. With the propose of bringing more profits to oversea customers by providing directly abroad. So we have changed our mind, from home to abroad, hope to give our customers more profit, and looking forward to more chance to make business.
Goods just received, we are very satisfied, a very good supplier, hope to make persistent efforts to do better.