Factory For Chlamydia Screen Test Seller - Diagnostic Kit(Colloidal Gold)for Human Chorionic Gonadotrophin – Baysen
Factory For Chlamydia Screen Test Seller - Diagnostic Kit(Colloidal Gold)for Human Chorionic Gonadotrophin – Baysen Detail:
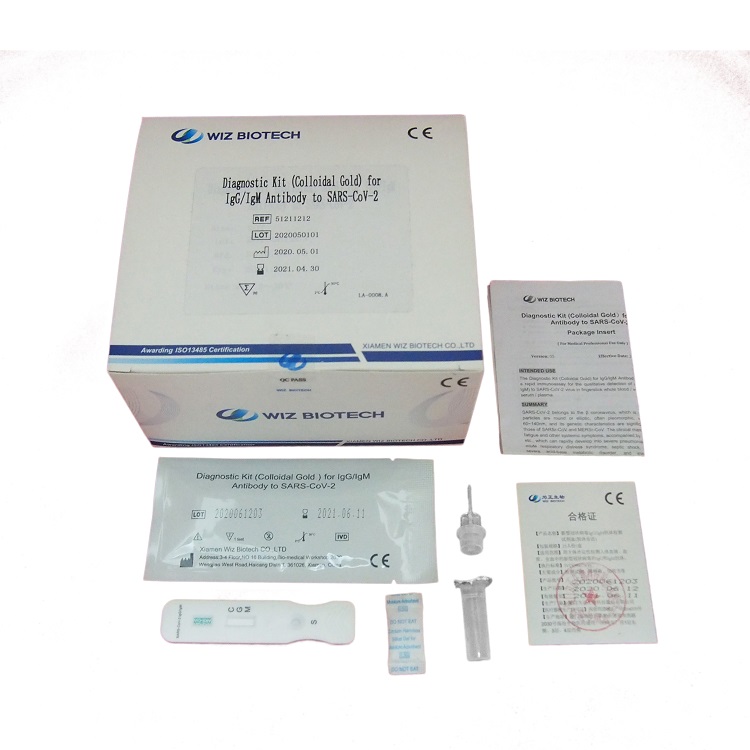
Diagnostic Kit(Colloidal Gold)for Human Chorionic Gonadotrophin
For in vitro diagnostic use only
Please read this package insert carefully prior to use and strictly follow the instructions. Reliability of assay results cannot be guaranteed if there are any deviations from the instructions in this package insert.
INTENDED USE
Diagnostic Kit(Colloidal Gold)for Human Chorionic Gonadotrophin is a colloidal gold immunochromatographic assay for the qualitative detection of human chorionic gonadotropin (HCG) levels in human serum and urine, it is used for early pregnancy diagnosis This test is a screening reagent. All positive sample must be confirmed by other methodologies. This test is intended for healthcare professional use only.
PACKAGE SIZE
1 kit /box, 10 kits /box, 25 kits,/box, 50 kits /box.
SUMMARY
HCG is a glycoprotein hormone secreted by the developing placenta after egg fertilization. HCG levels can be rapidly elevated in serum or urine as early as 1 to 2.5 weeks during pregnancy, and reach a peak in 8 week, than fall to medium level in 4 month, and maintained the level until the end of the pregnancy[1]. The Kit is a simple, visual qualitative test that detects HCG antigen in human serum or urine. The Diagnostic Kit is based on immunochromatography and can give a result within 15 minutes.
ASSAY PROCEDURE
1.Take out the test card from the foil bag, put it on the level table and mark it.
2.Discard the first two drops sample, add 3 drops (about 100μL) no bubble sample verticaly and slowly into sample well of the card with provided dispette, start timing.
3.The result should be read within 10-15 minutes, and it is invalid after 15 minutes.
Product detail pictures:

Related Product Guide:
Direct Reading of Bona Fide Barcode Assays for Diagnostics with Smartphone Apps | Psa Test Cost
Swell High-Protein Ice Cream at Trader Joe’s | Calprotectin Elisa Kit
That has a sound small business credit, great after-sales service and modern production facilities, we've earned an outstanding standing amid our buyers across the earth for Factory For Chlamydia Screen Test Seller - Diagnostic Kit(Colloidal Gold)for Human Chorionic Gonadotrophin – Baysen , The product will supply to all over the world, such as: Greenland, Toronto, Tanzania, Please really feel free to send us your requirements and we'll respond to you asap. We have got a professional engineering group to serve for your just about every detailed needs. Cost-free samples could be sent for you personally to understand much more information. In an effort to meet your requires, please really feel free to make contact with us. You may send us emails and contact us directly. Moreover, we welcome visits to our factory from around the globe for much better recognizing of our organization. nd items. In our trade with merchants of numerous countries, we usually adhere for the principle of equality and mutual benefit. It is actually our hope to market, by joint efforts, each trade and friendship to our mutual advantage. We look forward to getting your inquiries.
The company can think what our think, the urgency of urgency to act in the interests of our position, can be said this is a responsible company, we had a happy cooperation!







-3.jpg)