Diagnostic kit for IgM Antibody to Enterovirus 71 Colloidal Gold
Diagnostic kit for IgM Antibody to Enterovirus 71
Colloidal Gold
Production information
| Model Number | EV-71 | Packing | 25 Tests/ kit, 30kits/CTN |
| Name | Diagnostic kit for IgM Antibody to Enterovirus 71 Colloidal Gold | Instrument classification | Class I |
| Features | High sensitivity, Easy opeation | Certificate | CE/ ISO13485 |
| Accuracy | > 99% | Shelf life | Two Years |
| Methodology | Colloidal Gold | OEM/ODM service | Avaliable |
Test procedure
| 1 | Take the test device out of aluminum foil bag, place it on a flat tabletop and properlymark the sample. |
| 2 | Add 10uL of serum or plasma sample or 20uL of whole blood to sample hole, and then
drip 100uL (about 2-3 drops) of sample diluent to sample hole and start timing. |
| 3 | Result should be read within 10-15 minutes. Test result will be invalid after 15 minutes. |
Note: each sample shall be pipetted by clean disposable pipette to avoid cross contamination.
Intend Use
This kit is applicable to the in vitro quantitative detection on the content of IgM Antibody to Enterovirus 71 in human whole blood, serum or plasma and is mainly used for implementing auxiliary diagnosis of acute EV71 infection. This kit only provides the test result of IgM Antibody to Enterovirus 71 and the obtained result shall be analyzed in combination with other clinical information. It must only be used by healthcare professionals.

Summary
Feature:
• High sensitive
• result reading in 15 minutes
• Easy operation
• Factory direct price
• Do not need extra machine for result reading
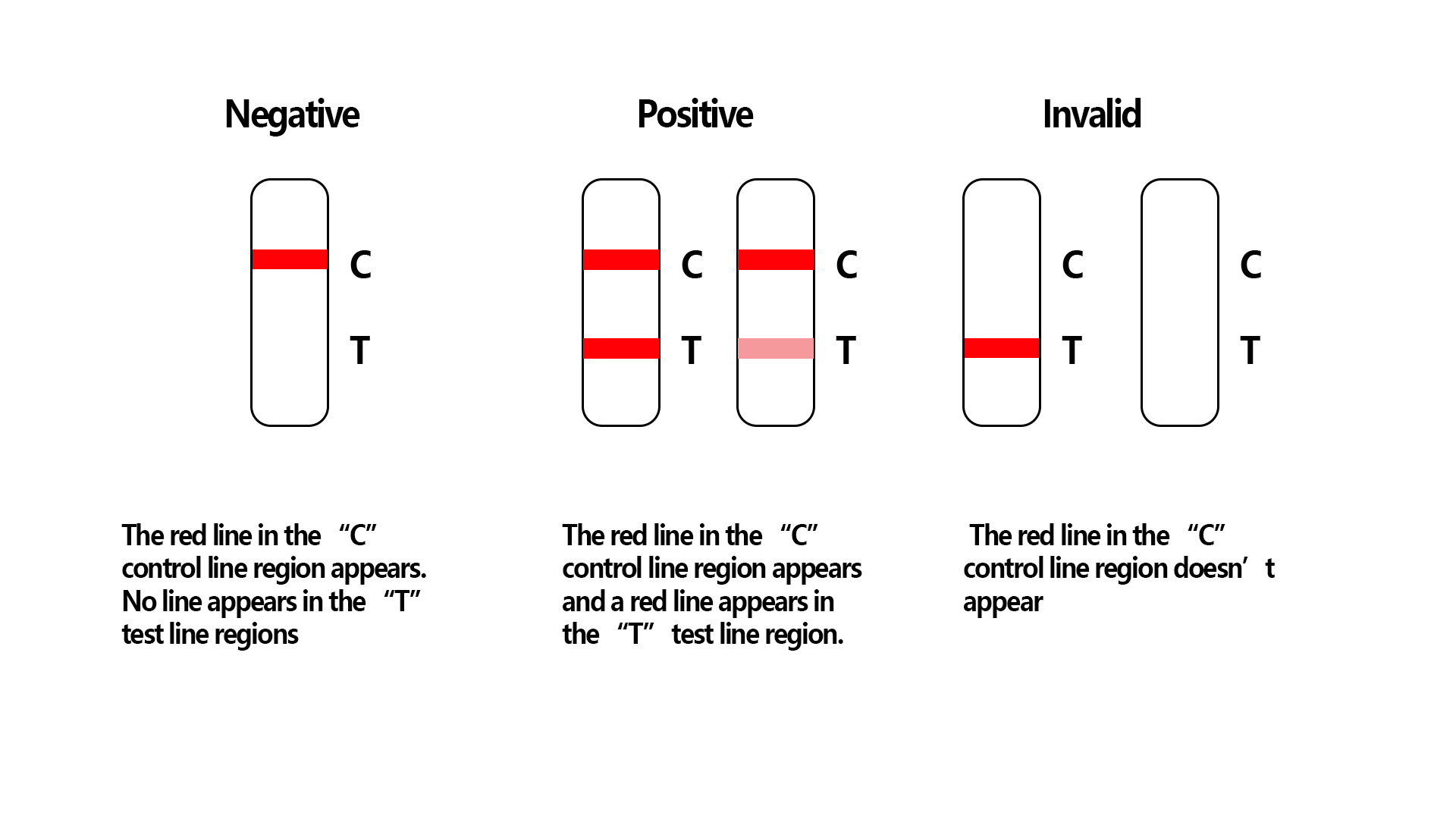
Result reading
The WIZ BIOTECH reagent test will be compared with the control reagent:
| Test result of wiz | Test result of reference reagents | Positive coincidence rate:99.39%(95%C.I.96.61%~99.89%) Negative coincidence rate:100%(95%C.I.97.63%~100%)
Total compliance rate: 99.69%(95%C.I.98.26%~99.94%) |
||
| Positive | Negative | Total | ||
| Positive | 162 | 0 | 162 | |
| Negative | 1 | 158 | 159 | |
| Total | 163 | 158 | 321 | |
You may also like: