Chinese Professional Mycoplasma Test - Diagnostic Kit(LATEX)for Rotavirus Group A – Baysen
Chinese Professional Mycoplasma Test - Diagnostic Kit(LATEX)for Rotavirus Group A – Baysen Detail:
Diagnostic Kit(LATEX)for Rotavirus Group A
For in vitro diagnostic use only
Please read this package insert carefully prior to use and strictly follow the instructions. Reliability of assay results cannot be guaranteed if there are any deviations from the instructions in this package insert.
INTENDED USE
Diagnostic Kit(LATEX)for Rotavirus Group A is suitable for qualitative detection of Rotavirus Group A antigen in human fecal samples. This test is intended for healthcare professional use only. Meanwhile, this test is used for the clinical diagnosis of infantile diarrhea in patients with Rotavirus Group A infection.
PACKAGE SIZE
1 kit /box, 10 kits /box, 25 kits,/box, 50 kits /box.
SUMMARY
Rotavirus is classified as a rotavirus genus of the exenteral virus, which has a spherical shape with a diameter of about 70nm. Rotavirus contains 11 segments of double-stranded RNA. The rotavirus can be seven groups (a-g) based on antigenic differences and gene characteristics. Human infections of group A, group B and C group rotavirus have been reported. Rotavirus Group A is the important cause of severe gastroenteritis in children worldwide[1-2].
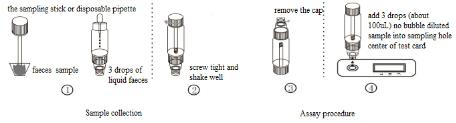
ASSAY PROCEDURE
1.Take out the sampling stick, inserted into the faeces sample, then put the sampling stick back, screw tight and shake well, repeat the action 3 times. Or using the sampling stick picked about 50mg faeces sample, and put in a faeces sample tube containing sample dilution, and screw tightly.
2.Use disposable pipette sampling take the thinner faeces sample from the diarrhea patient, then add 3 drops (about 100uL) to the fecal sampling tube and shake well, put aside.
3.Take out the test card from the foil bag, put it on the level table and mark it.
4.Remove the cap from the sample tube and discard the first two drops diluted sample, add 3 drops (about 100uL) no bubble diluted sample verticaly and slowly into sample well of the card with provided dispette, start timing.
5.The result should be read within 10-15 minutes, and it is invalid after 15 minutes.
Product detail pictures:



Related Product Guide:
MRI scans are sparing men painful prostate cancer investigations | P24 Test Strips
PSA testing for prostate cancer | Health Information | Cpn-Igm
To be the stage of realizing dreams of our employees! To build a happier, more united and more professional team! To reach a mutual benefit of our customers, suppliers, the society and ourselves for Chinese Professional Mycoplasma Test - Diagnostic Kit(LATEX)for Rotavirus Group A – Baysen , The product will supply to all over the world, such as: Slovakia, Puerto Rico, Marseille, Corporate goal: Customers' satisfaction is our goal, and sincerely hope to establish long-terms stable cooperative relations with customers to jointly develop the market. Building brilliant tomorrow together!Our company regards "reasonable prices, efficient production time and good after-sales service" as our tenet. We hope to cooperate with more customers for mutual development and benefits. We welcome potential buyers to contact us.
The factory workers have rich industry knowledge and operational experience, we learned a lot in working with them,we are extremely grateful that we can encount a good company has excellent wokers.