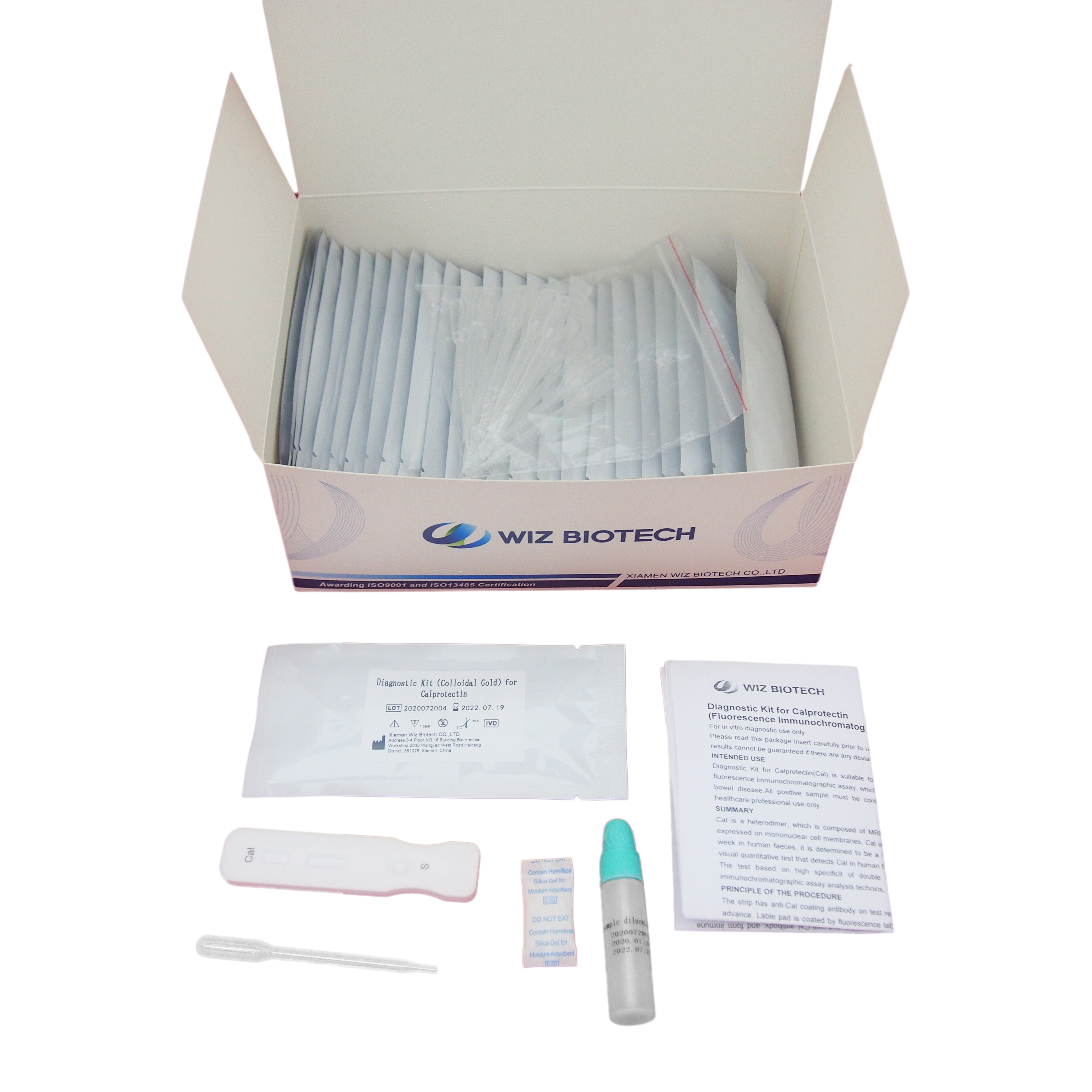
China OEM Faecal Calprotectin Test Results - Diagnostic Kit(Colloidal Gold)for IgM Antibodv to Chlamydia Pneumoniae – Baysen
China OEM Faecal Calprotectin Test Results - Diagnostic Kit(Colloidal Gold)for IgM Antibodv to Chlamydia Pneumoniae – Baysen Detail:
Diagnostic Kit(Colloidal Gold)for IgM Antibodv to Chlamydia Pneumoniae
For in vitro diagnostic use only
Please read this package insert carefully prior to use and strictly follow the instructions. Reliability of assay results cannot be guaranteed if there are any deviations from the instructions in this package insert.
INTENDED USE
Diagnostic Kit(Colloidal Gold)for IgM Antibodv to Chlamydia Pneumoniae is a colloidal gold immunochromatographic assay for the qualitative determination of IgM Antibody to Chlamydia Pneumoniae (Cpn-IgM) in human whole blood,serum or plasma, it acts as chlamydia pneumonia infection auxiliary diagnosis reagent in clinical diagnosis. Meanwhile it is a screening reagent. All positive sample must be confirmed by other methodologies.This test is intended for healthcare professional use only.
PACKAGE SIZE
1 kit /box, 10 kits /box, 25 kits,/box, 50 kits /box
SUMMARY
Chlamydia pneumoniae is an important pathogen of respiratory infection, it can cause upper respiratory tract infection,such as sinusitis, otitis and pharyngitis, and lower respiratory tract infection, such as bronchitis and pneumonia.The Diagnostic Kit is a simple, visual qualitative test that detects Cpn-Igm in human whole blood, serum or plasma. The Diagnostic Kit is based on immunochromatography and can give a result within 15 minutes.
Applicable instrument
Except visual inspection, the kit can be matched with Continuous immune analyzer WIZ-A202 of Xiamen Wiz Biotech Co., Ltd
ASSAY PROCEDURE
The WIZ-A202 test procedure see the instruction of Continuous immune analyzer. Visual test procedure is as follows
1.Take out the test card from the foil bag, put it on the level table and mark it;
2.Add 10μl serum or plasma sample or 20ul whole blood sample to sample well of the card with provided dispette, then add 100μl (about 2-3 drop) sample diluent; start timing;
3.Wait for a minimum 10-15 minutes and read the result, the result is invalid after 15 minutes.
Product detail pictures:

Related Product Guide:
PSA tests are still a good way to detect prostate cancer | Diagnostic Kit For Isoenzyme Mb Of C Reatine Kinase
Cancer doctors give new PSA testing advice for some men | P24 Test Strips
We have been ready to share our knowledge of internet marketing worldwide and recommend you suitable merchandise at most aggressive rates. So Profi Tools present you very best price of money and we are ready to develop alongside one another with China OEM Faecal Calprotectin Test Results - Diagnostic Kit(Colloidal Gold)for IgM Antibodv to Chlamydia Pneumoniae – Baysen , The product will supply to all over the world, such as: Guatemala, Seattle, Frankfurt, Our products are exported worldwide. Our customers are always satisfied with our reliable quality, customer-oriented services and competitive prices. Our mission is "to continue to earn your loyalty by dedicating our efforts to the constant improvement of our products and services in order to ensure the satisfaction of our end-users, customers, employees, suppliers and the worldwide communities in which we cooperate".
On this website, product categories is clear and rich, I can find the product I want very quickly and easily, this is really very good!