18 Years Factory Hcv Test Strip - Professional Factory for Lansionbio CE ISO Approved Prl Rapid Test Kit – Baysen
18 Years Factory Hcv Test Strip - Professional Factory for Lansionbio CE ISO Approved Prl Rapid Test Kit – Baysen Detail:
The really abundant projects management experiences and 1 to just one provider model make the high importance of business enterprise communication and our easy understanding of your expectations for Professional Factory for Lansionbio CE ISO Approved Prl Rapid Test Kit, With us your money in protected your organization in safe . Hope we are able to be your trustworthy supplier in China . Looking ahead for your cooperation .
The really abundant projects management experiences and 1 to just one provider model make the high importance of business enterprise communication and our easy understanding of your expectations for China Prl Test Kit Manufacturing and Antigen Rapid Test Kit, We always stick to the tenet of “sincerity, high quality, high efficiency, innovation”. With years of efforts, now we have established friendly and stable business relationships with worldwide customers. We welcome any of your inquiries and concerns for our products, and we have been sure that we’ll provide just what you want, as we always believe that your satisfaction is our success.
FOB Brochure


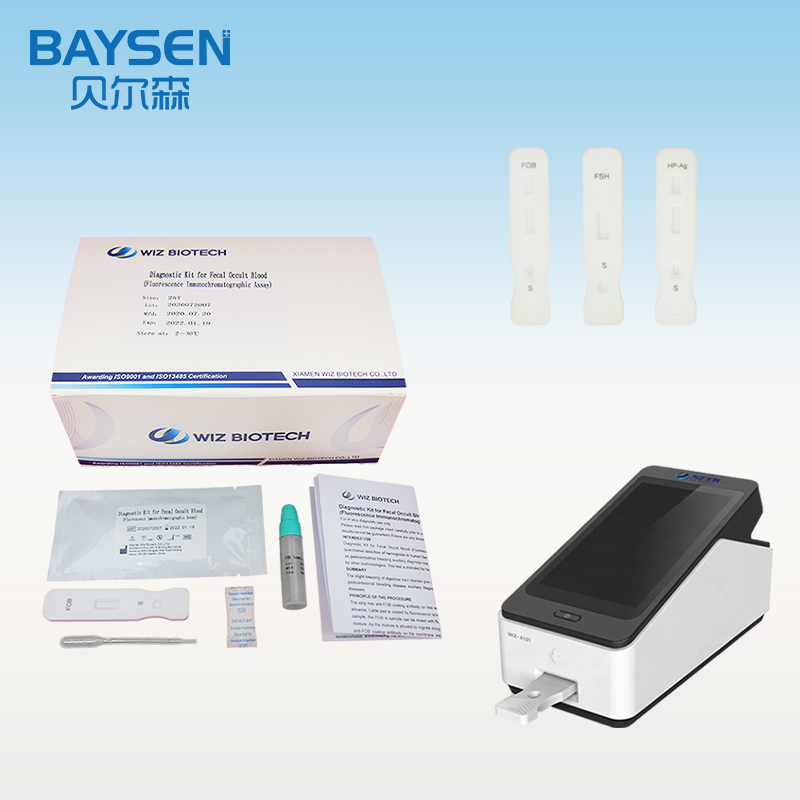
PRINCIPLE AND PROCEDURE OF FOB TEST
Princple:
The strip has anti-FOB coating antibody on test region, which is fastened to membrane chromatography in advance. Lable pad is coated by fluorescence labeled anti-FOB antibody in advance. When testing positive sample, the FOB in sample can be mixed with fluorescence labeled anti-FOB antibody, and form immune mixture. As the mixture is allowed to migrate along the test strip, the FOB conjugate complex is captured by anti-FOB coating antibody on the membrane and forms complex. The fluorescence intensity is positively correlated with the FOB content. The FOB in sample can be detected by fluorescence immunoassay analyzer.
Test Procedure:
1.Lay aside all reagents and samples to room temperature.
2.Open the Portable Immune Analyzer(WIZ-A101), enter the account password login according to the operation method of the instrument, and enter the detection interface.
3.Scan the dentification code to confirm the test item.
4.Take out the test card from the foil bag.
5.Insert the test card into the card slot, scan the QR code, and determine the test item.
6.Remove the cap from the sample tube and discard the first two drops diluted sample, add 3 drops (about 100uL) no bubble diluted sample verticaly and slowly into sample well of the card with provided dispette.
7.Click the “standard test” button, after 15 minutes, the instrument will automatically detect the test card, it can read the results from the display screen of the instrument, and record/print the test results.
8.Refer to the instruction of Portable Immune Analyzer(WIZ-A101).

You May like
SARS-CoV-2 Antigen Rapid Test(Colloidal Gold)
WIZ-A101 Portable Immune Analyzer
Diagnostic Kit for Follicle-Stimulating Hormone (Fluorescence Immunochromatographic Assay)
About Us

Xiamen Baysen Medical Tech limited is a high biological enterprise which devotes itself to filed of fast diagnostic reagent and integrates research and development, production and sales into a whole. There are many advanced research staffs and sales managers in the company, all of them are have rich working experience in china and international biopharmaceutical enterprise.
Certificate display

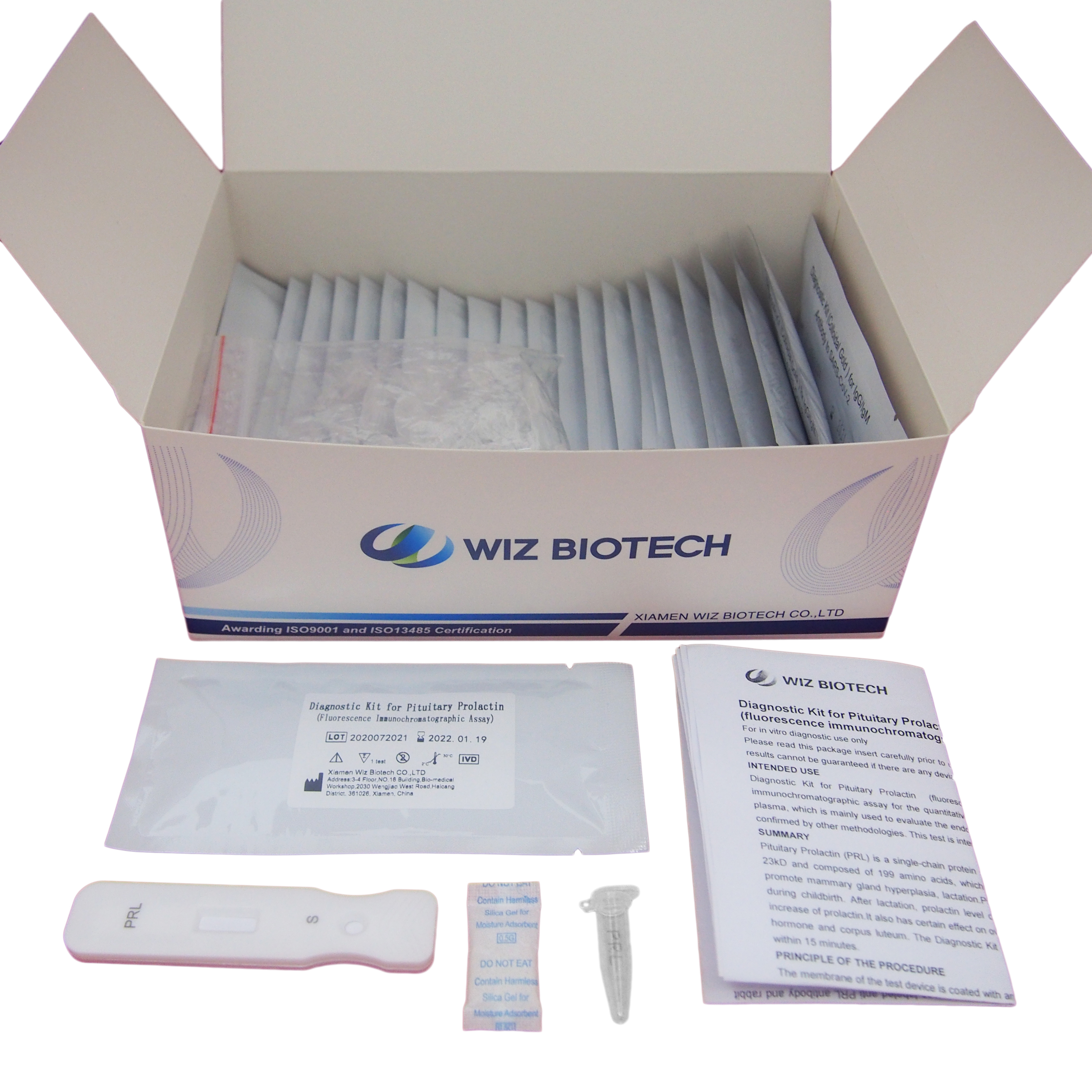
Product detail pictures:

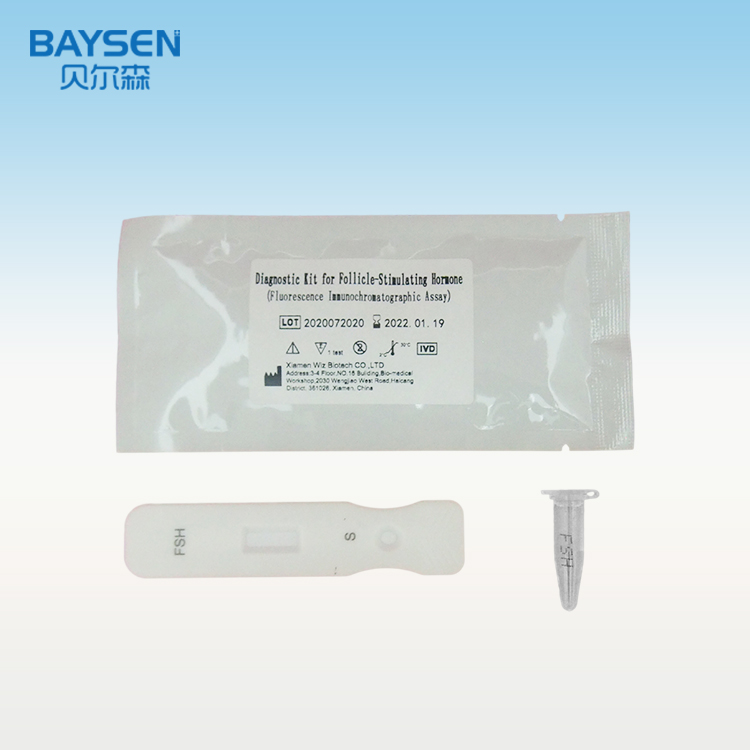
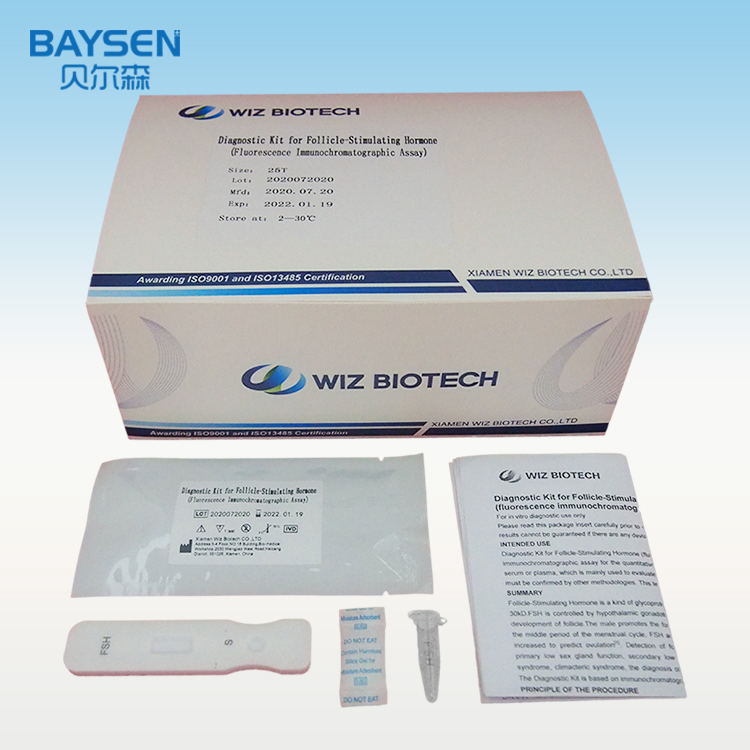
Related Product Guide:
Cancer doctors give new PSA testing advice for some men | Cpn-Igm
Silencing MicroRNA-155 Attenuates Cardiac Injury and Dysfunction in Viral Myocarditis via Promotion of M2 Phenotype Polarization of Macrophages | Calprotectin Elisa Kit
We support our prospective buyers with ideal top quality merchandise and superior level provider. Becoming the specialist manufacturer in this sector, we have now attained abundant practical expertise in producing and managing for 18 Years Factory Hcv Test Strip - Professional Factory for Lansionbio CE ISO Approved Prl Rapid Test Kit – Baysen , The product will supply to all over the world, such as: European, Hongkong, Gambia, We welcome you to visit our company, factory and our showroom displayed various products that will meet your expectation, meanwhile, it is convenient to visit our website, our sales staff will try their efforts to provide you the best service. If you need more information, please do not hesitate to contact us by E-mail or telephone.
We are really happy to find such a manufacturer that ensuring product quality at the same time the price is very cheap.














